Contents:
Naming organic molecules might seem tricky at first, but it’s a crucial skill for any chemist. Being able to convert names into structures, and vice-versa, is key to mastering organic chemistry. The beauty of the IUPAC naming system is that it’s unambiguous. If you follow the rules, you will describe a completely unique molecule without the need to draw it!
What is the IUPAC naming system?
The IUPAC naming system is a set of rules used to give every organic compound a unique name based on its structure. It was developed by the International Union of Pure and Applied Chemistry (IUPAC) to standardise the naming of organic compounds worldwide.
Steps for naming organic compounds
Step 1: Find the longest chain
Look at your molecule. What is the longest continuous chain of carbon atoms? This chain forms the ‘backbone’ of the molecule, and its length determines the base name, or prefix. The table below outlines the prefixes you need to know. Learn them!
Note that exam boards differ here. AQA requires you to know the names of the first 6 carbon atoms, but OCR and Edexcel expect you to learn up to 10 carbon atoms.
Sometimes, cheeky examiners try to disguise the longest chain. Make sure you find the longest possible chain, even if it’s not in a straight line.
Step 2: Look for side chains
Anything attached to the backbone is also known as a substituent. A common substituent is a ‘side chain’, which is a group of carbon atoms that is not part of the main chain but bonded to it.
A side chain could be a single carbon atom or a longer chain. Count how many carbon atoms are in the side chain, then use the same prefix as before to name it, but with an “-yl” ending.
1 carbon: Methyl
2 carbons: Ethyl
3 carbons: Propyl
4 carbons: Butyl, and so on.
Now we have identified the side chain, the next step is to describe where it goes.
The two molecules shown below are different because the methyl side chain is attached at different positions on the backbone.
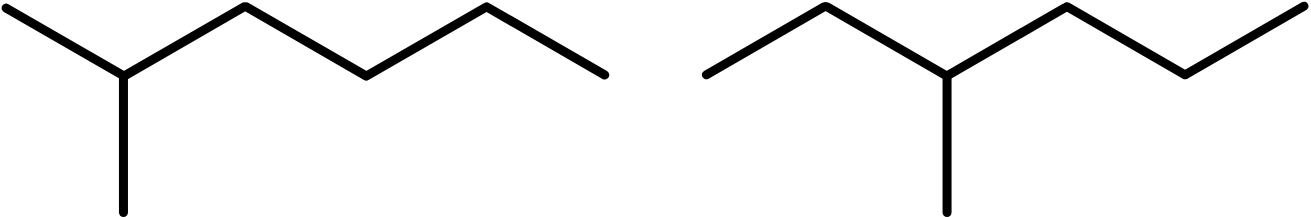
The trick here is to number the carbon atoms in the backbone, starting at 1. In the left example, the methyl group is in the 2nd position. But, if we number from the other direction, it’s in the 5th position. The rule is that you always go with the smaller number. So this molecule is called 2-methylhexane.
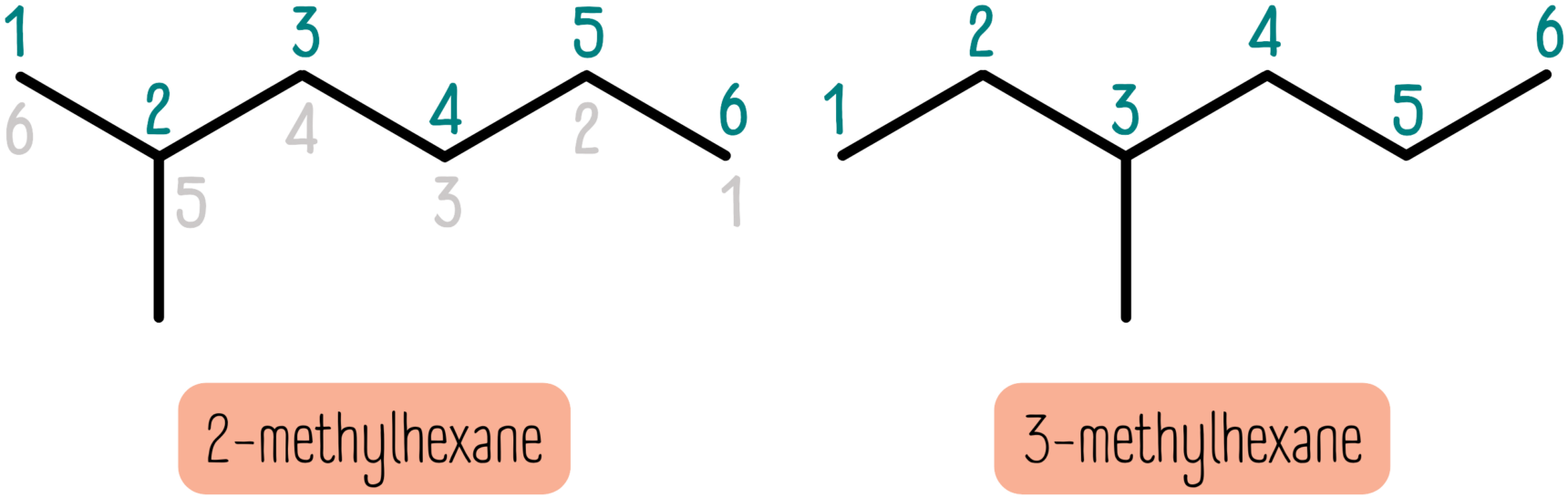
The last step here is to describe how many side chains there are. If there are 2 methyl groups attached to the backbone, we have to describe this somehow. We use the prefixes di-, tri- and tetra- for this.
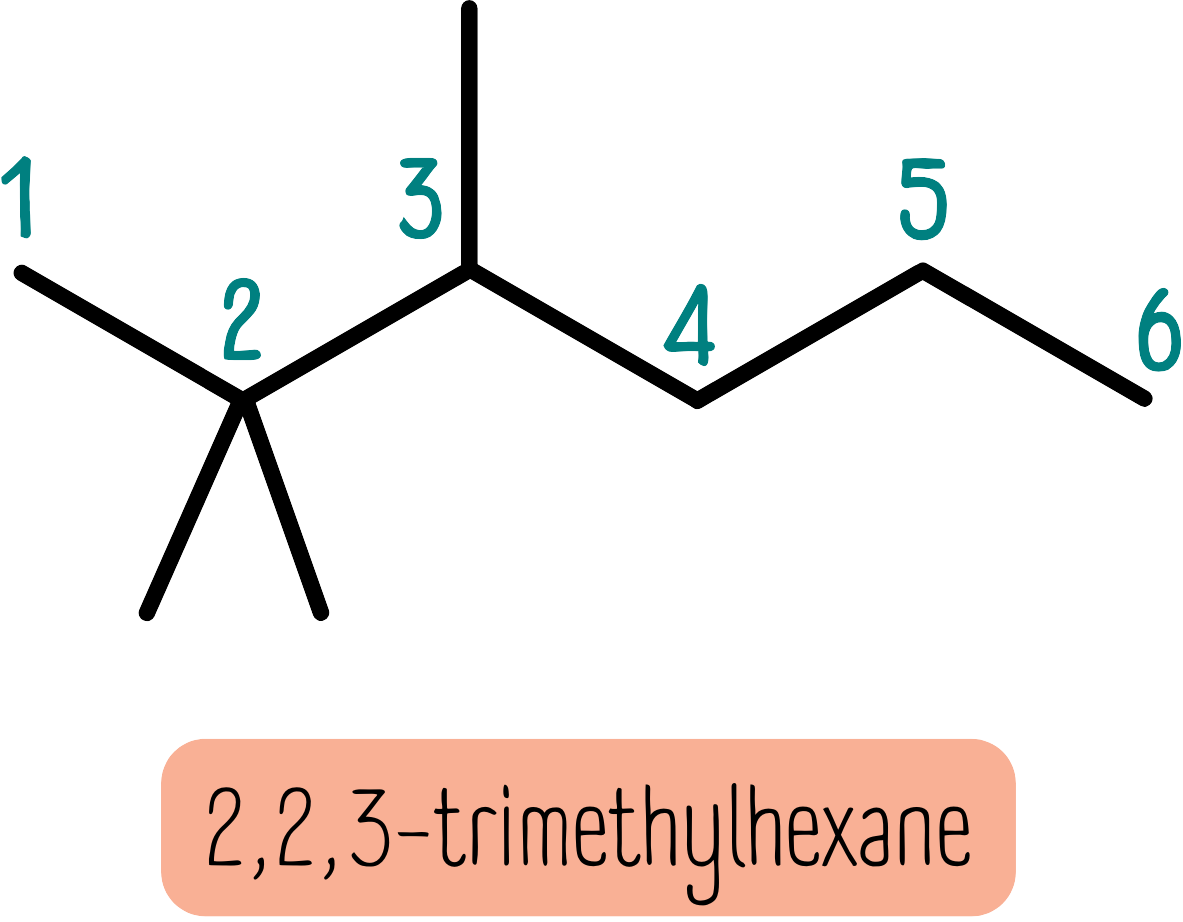
The example below has a backbone of 6 carbon atoms and 3 substituent methyl groups, so it is a trimethylhexane. However, this does not describe a unique molecule until we say where the 3 methyl groups are attached. This molecule is 2,2,3-trimethylhexane.
Step 3: Identify other substituents
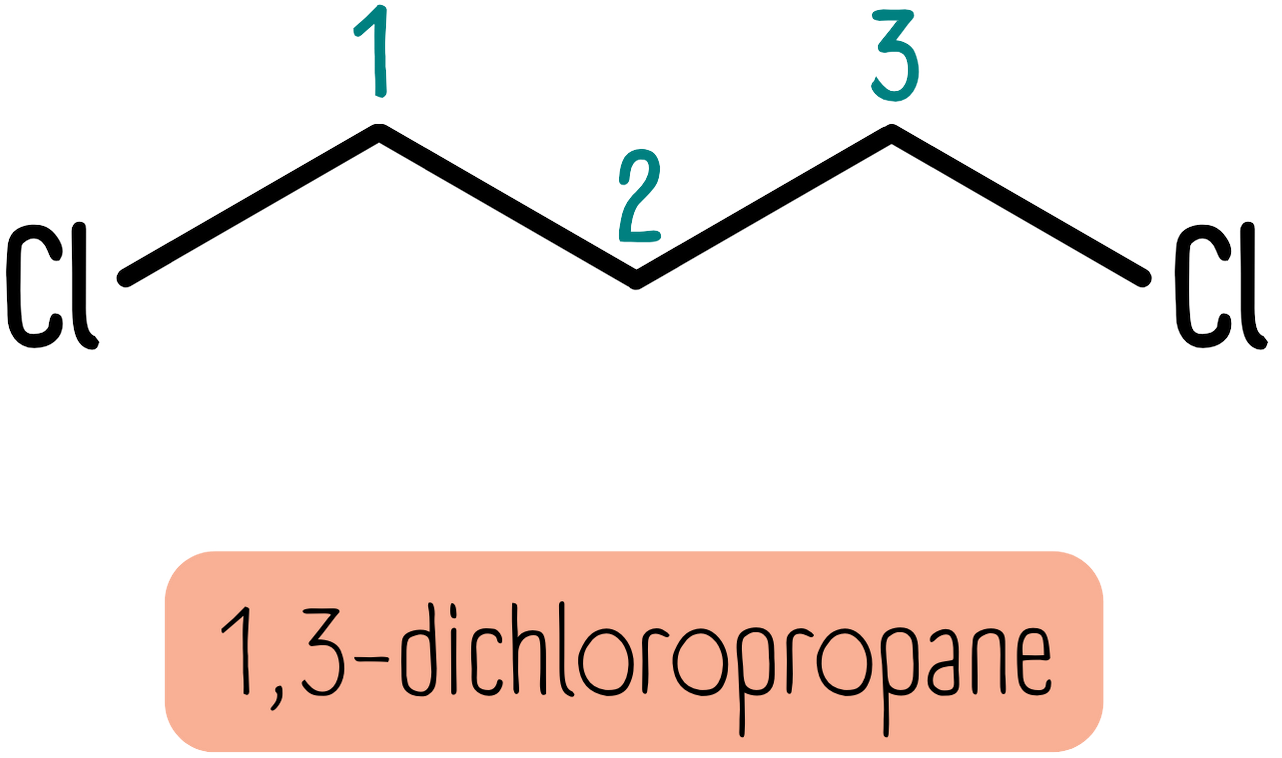
There are other substituents which you can easily look for. Common substituents are halides (fluoro, chloro, bromo etc.). Again, you must describe how many there are and where they are. The molecule below is 1,3-dichloropropane:
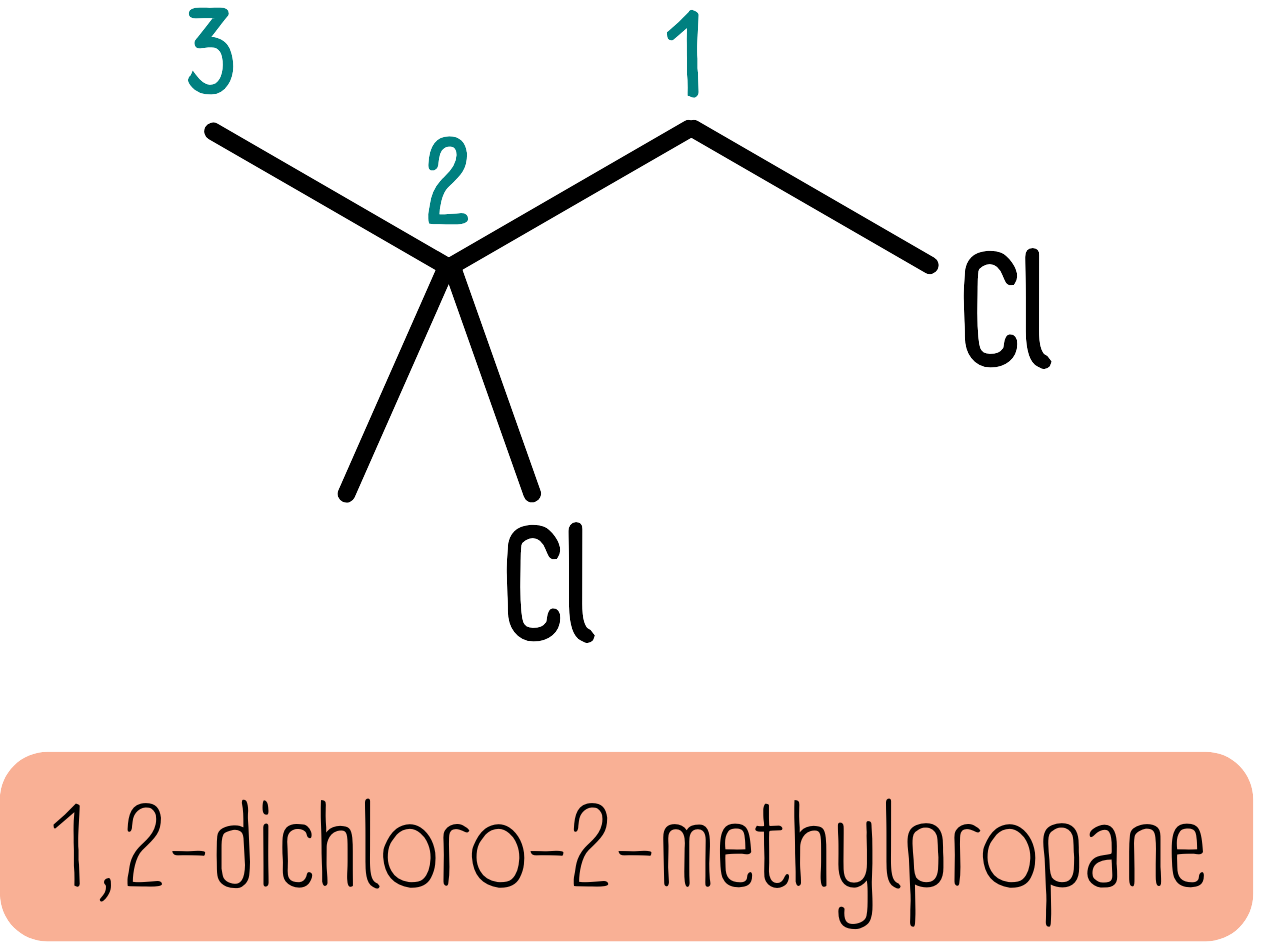
If there are different substituents, these must be written in alphabetical order (ignoring di, tri, tetra, etc). So the molecule below is 1,2-dichloro-2-methylpropane because “chloro” is written before “methyl”.
Alcohols are named in a similar way, but we use the suffix -ol and a number to indicate the position. The molecule below is 2-methylpropane-1,2-diol.
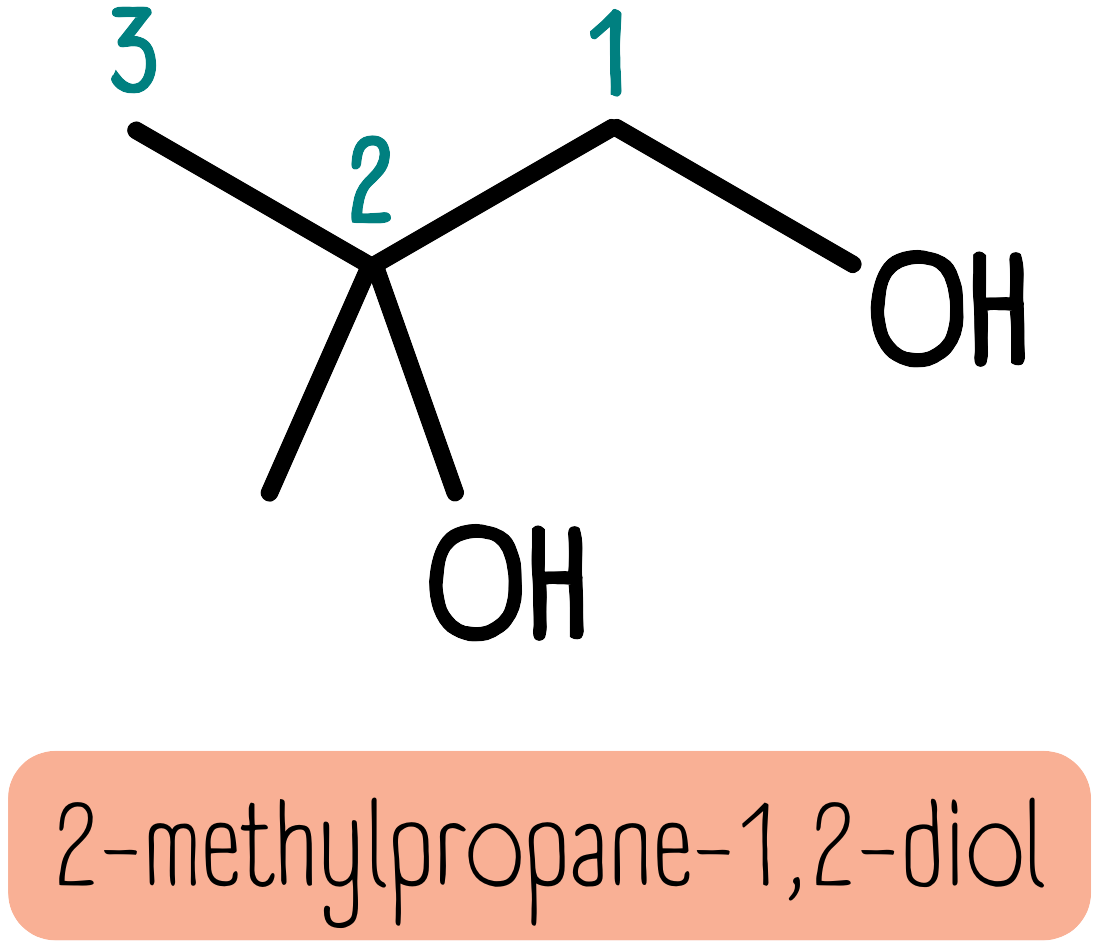
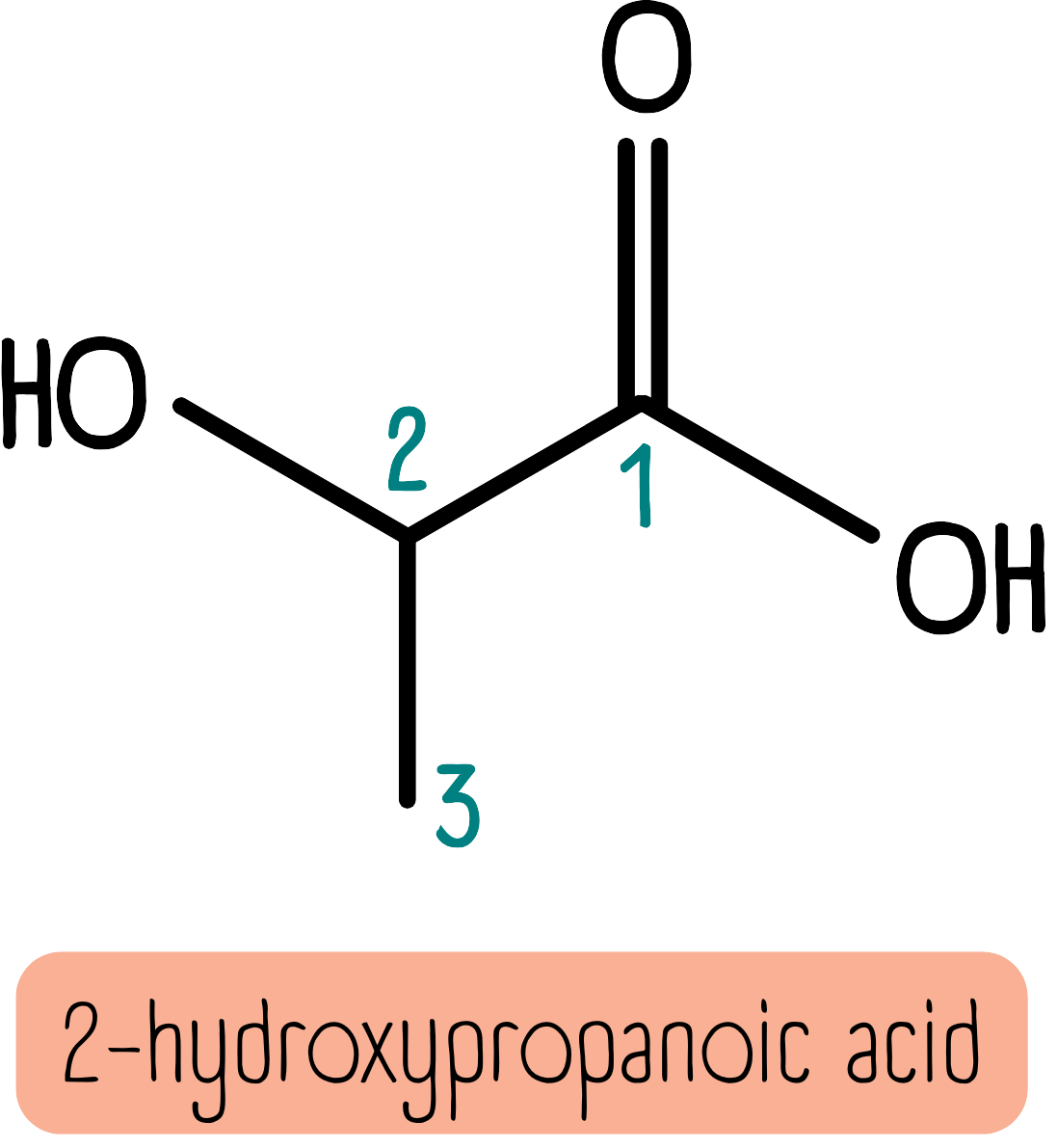
The final rule for alcohols is that if there is a carbonyl group present, the alcohol becomes a hydroxy group and we add the suffix -hydroxy. The molecule below is 2-hydroxypropanoic acid.
Other substituents
You will come across a number of other functional groups in A Level Chemistry. Here is how you name a few of them.
Carbonyl compounds
The carbonyl group appears in a few different functional groups.
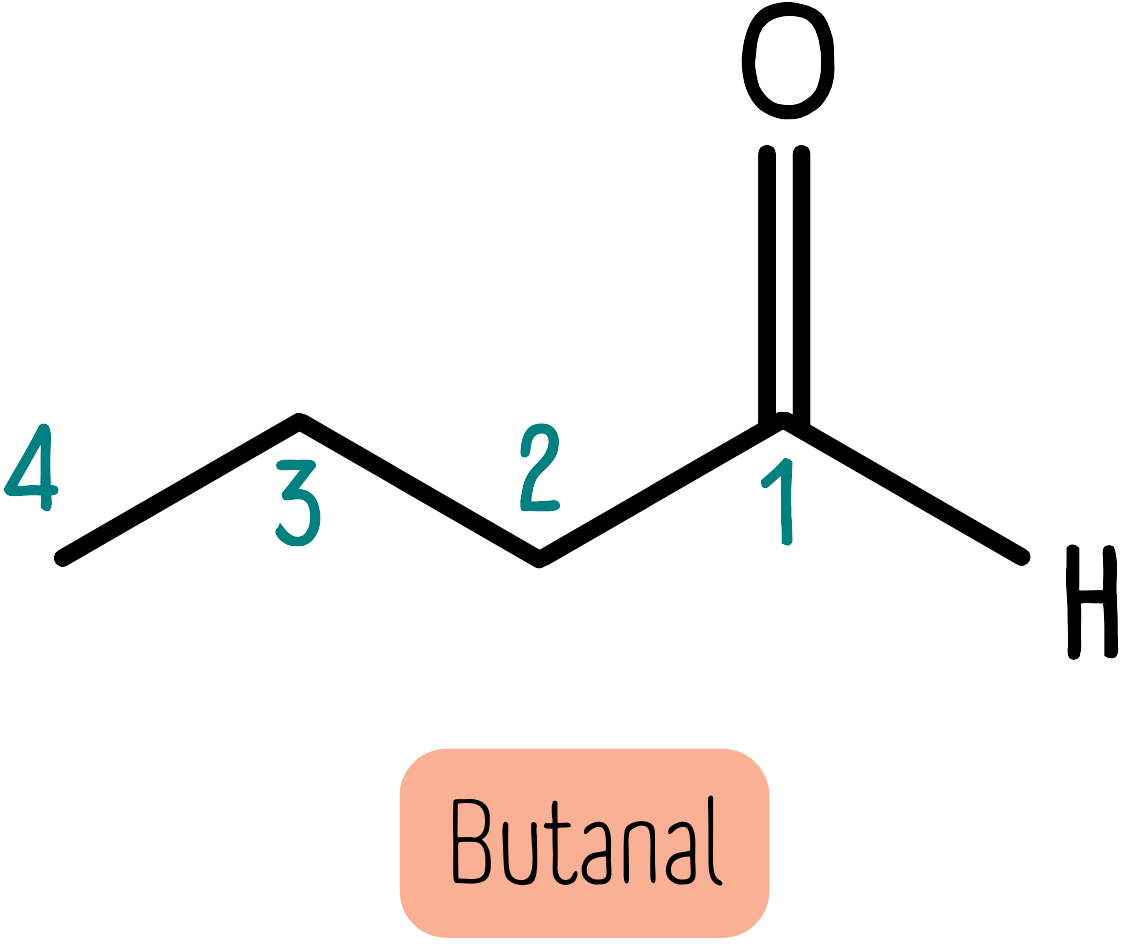
Aldehydes are characterised by a carbonyl group at the end of the molecule. They take priority and always end with the suffix -al. For example, the molecule below is butanal.
When numbering substituents, the carbon attached to the carbonyl is always the “number 1” carbon atom.
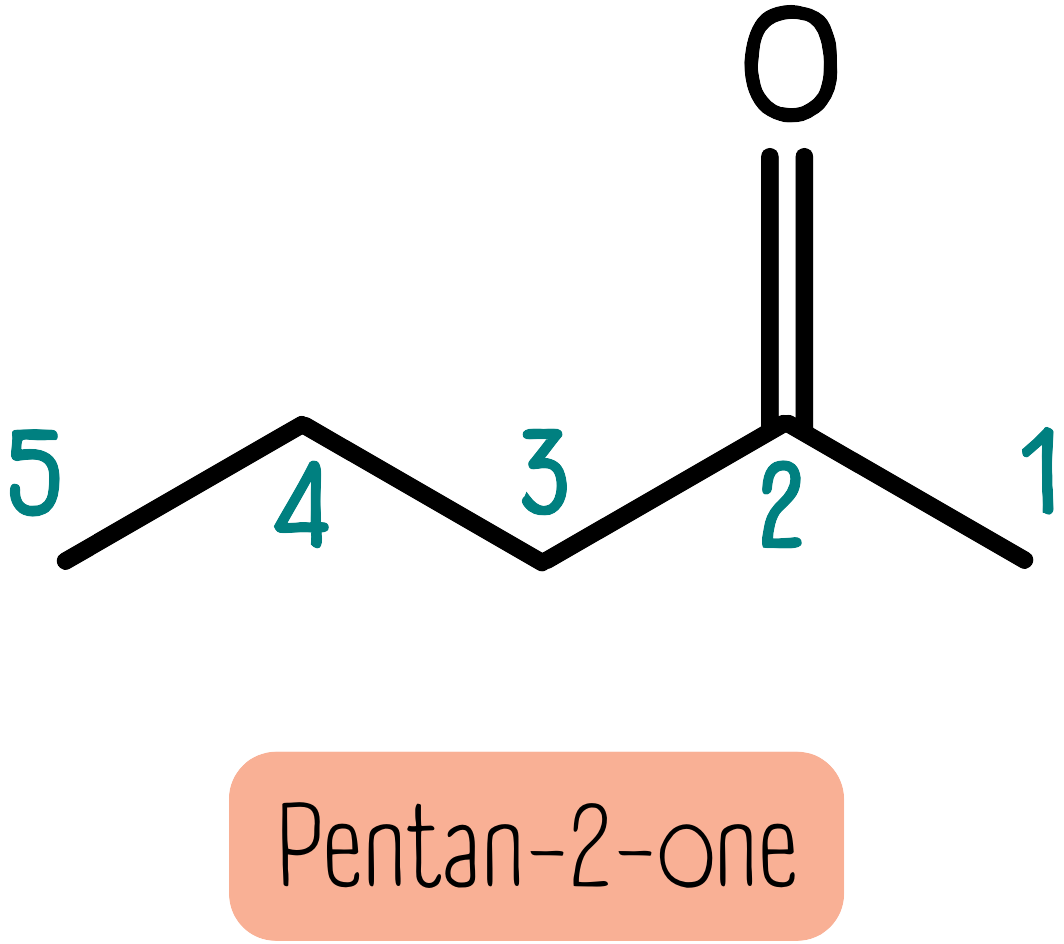
Ketones have a carbonyl group located within the carbon chain. They are named with the suffix -one. This usually needs a number to describe where the carbonyl is within the carbon chain. For instance, the molecule shown below is pentan-2-one, where the carbonyl group is on the second carbon.
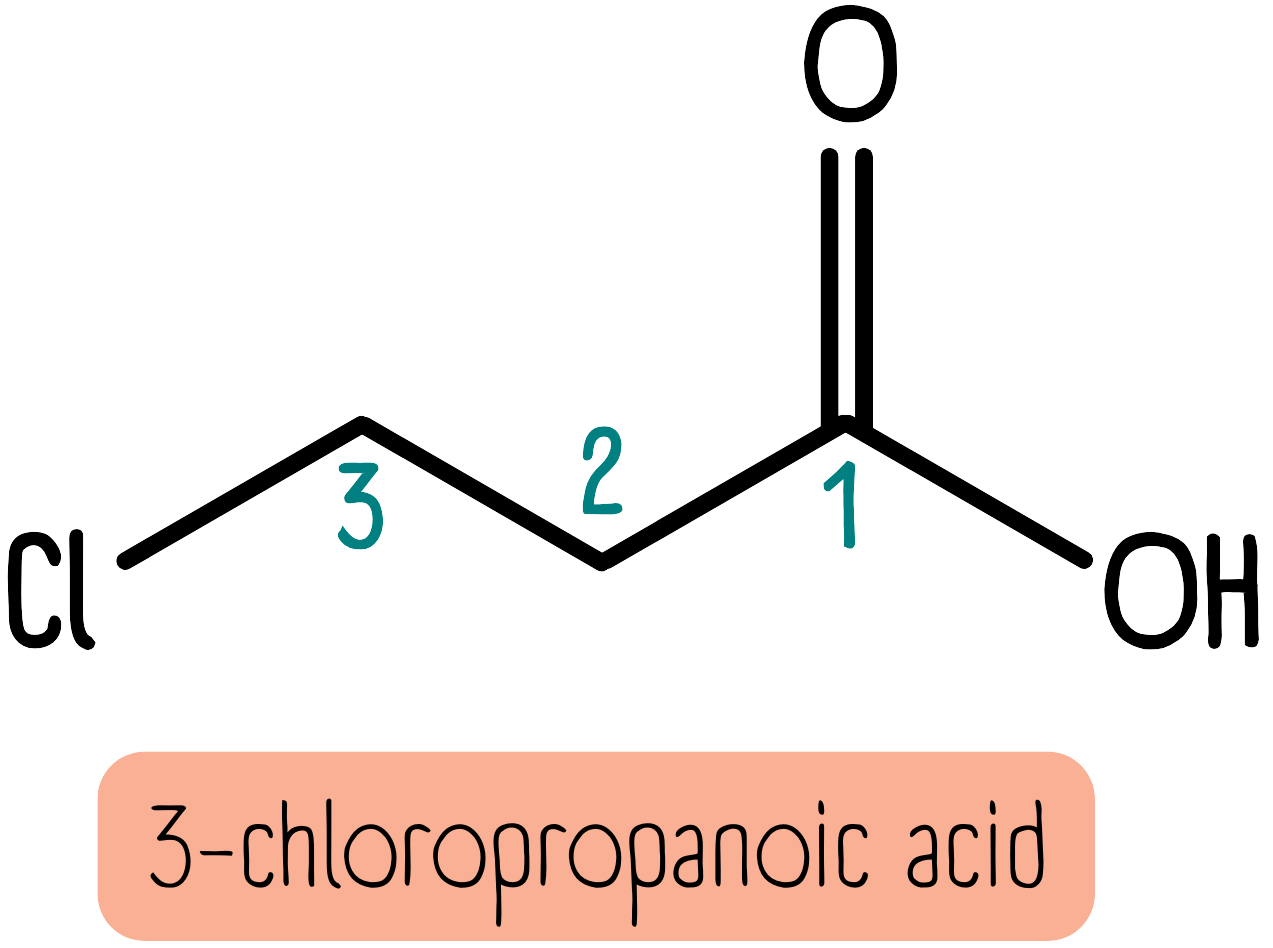
Carboxylic acids are characterised by a carboxyl group made up of a carbonyl group at the end of the molecule bonded to a hydroxy group. Carboxylic acids have the suffix -oic acid. The carboxyl group is always at the “number 1” end of the molecule. The molecule below is 3-chloropropanoic acid:
Amines
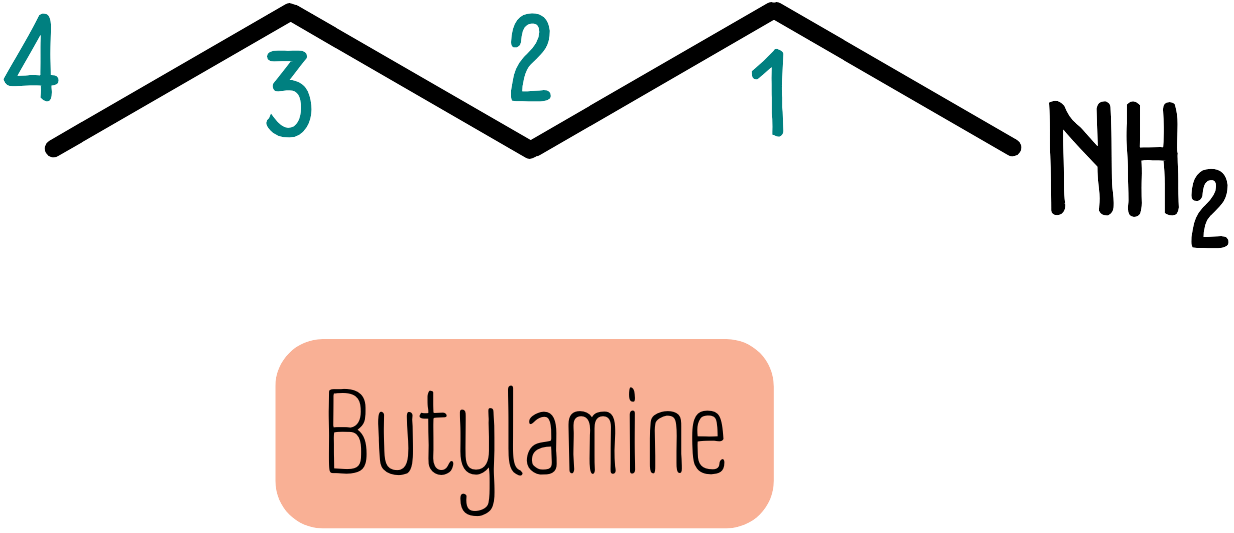
Primary amines are simple. If the amine group is on the end of a chain, simply add the suffix -amine. Butylamine is shown below:
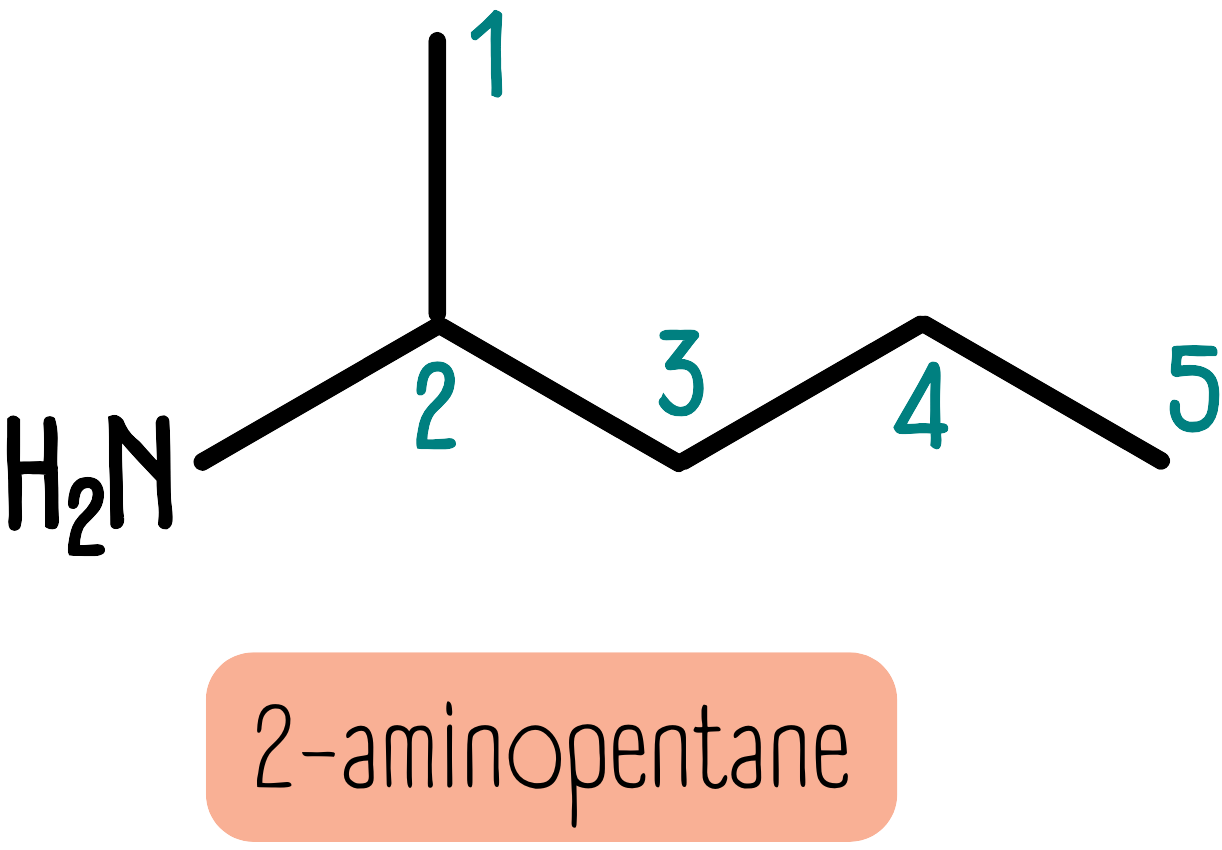
If the amine group is on a different position than the number 1, we name the molecule with the prefix amino-. We have to add a number at the front to indicate the carbon to which the amine group is attached. The molecule below is 2-aminopentane.
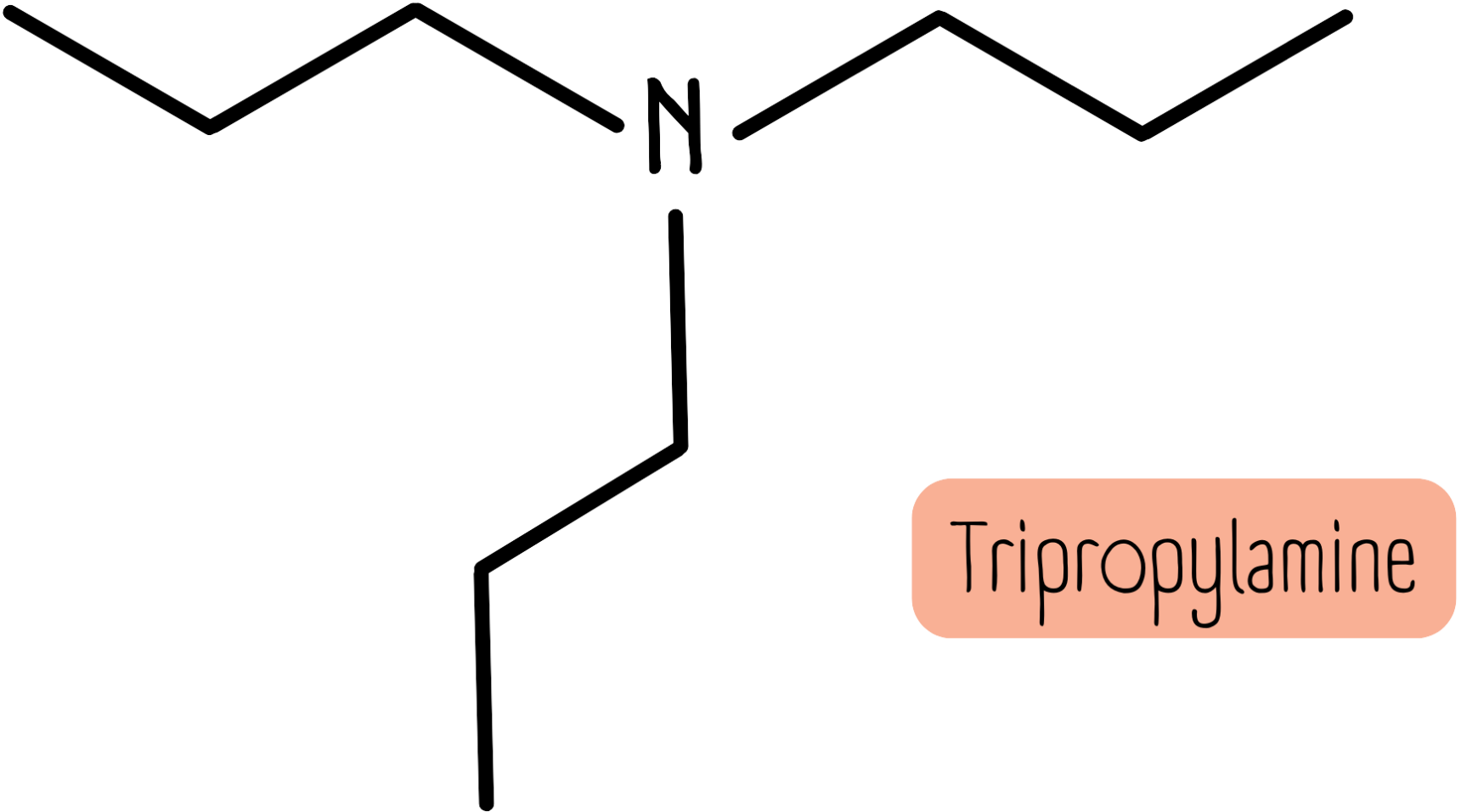
Secondary or tertiary amines with the same group attached are named as above, with the prefix di- or tri- to indicate the number of alkyl groups. The molecule below is tripropylamine.
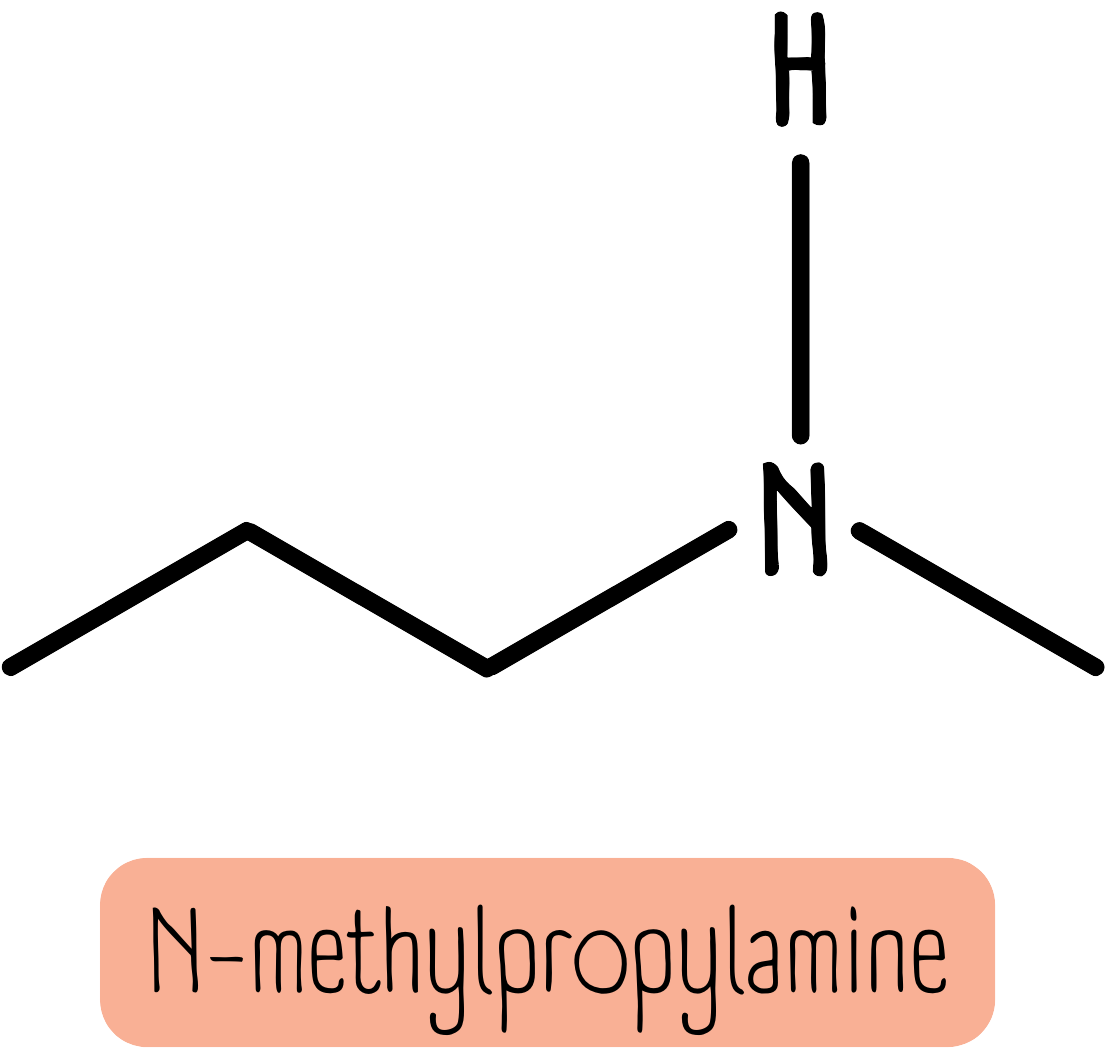
When the alkyl groups are different, we use the prefix N- to show an amine with an extra chain. The smaller chain is described first.
For example, the molecule below is N-methylpropylamine. You could think of this as a propylamine molecule with a smaller methyl substituent group attached.
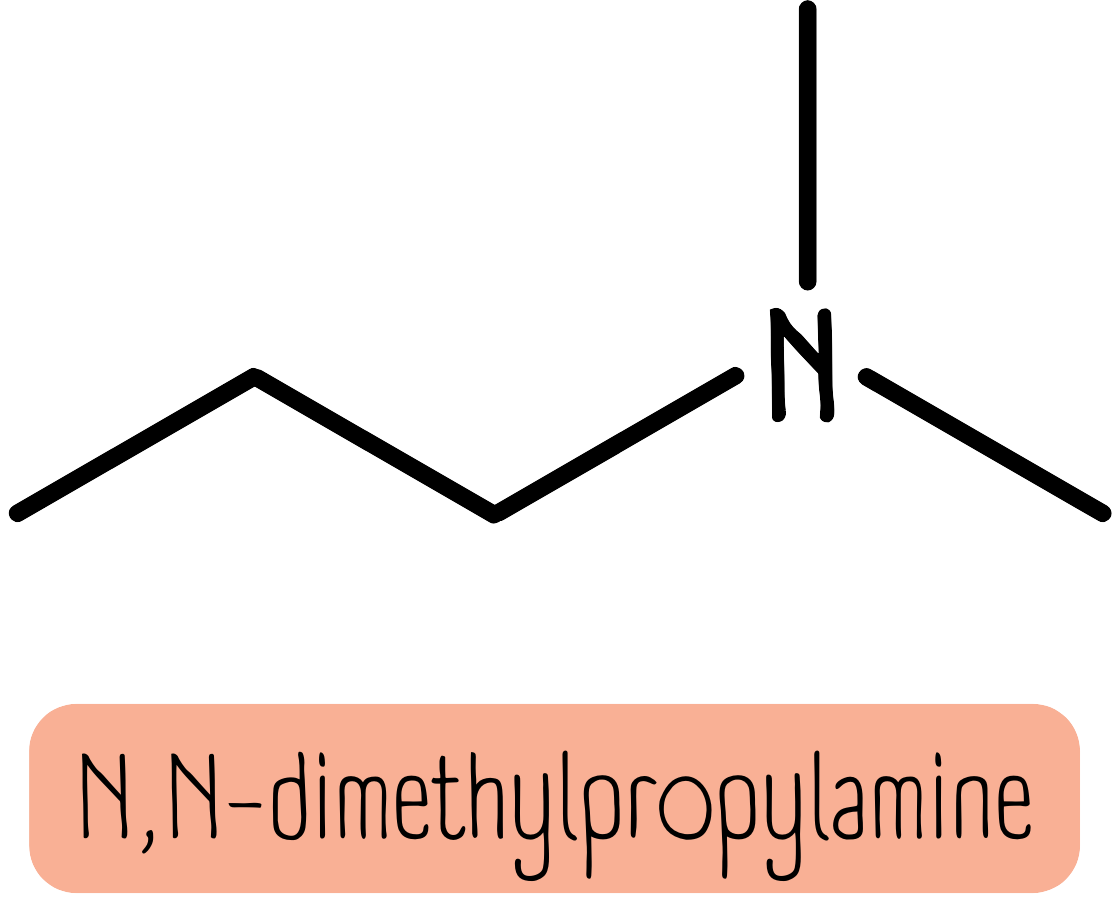
When there are 2 extra groups, we use the prefix N,N-. The molecule below is N,N-dimethylpropylamine.
Amides
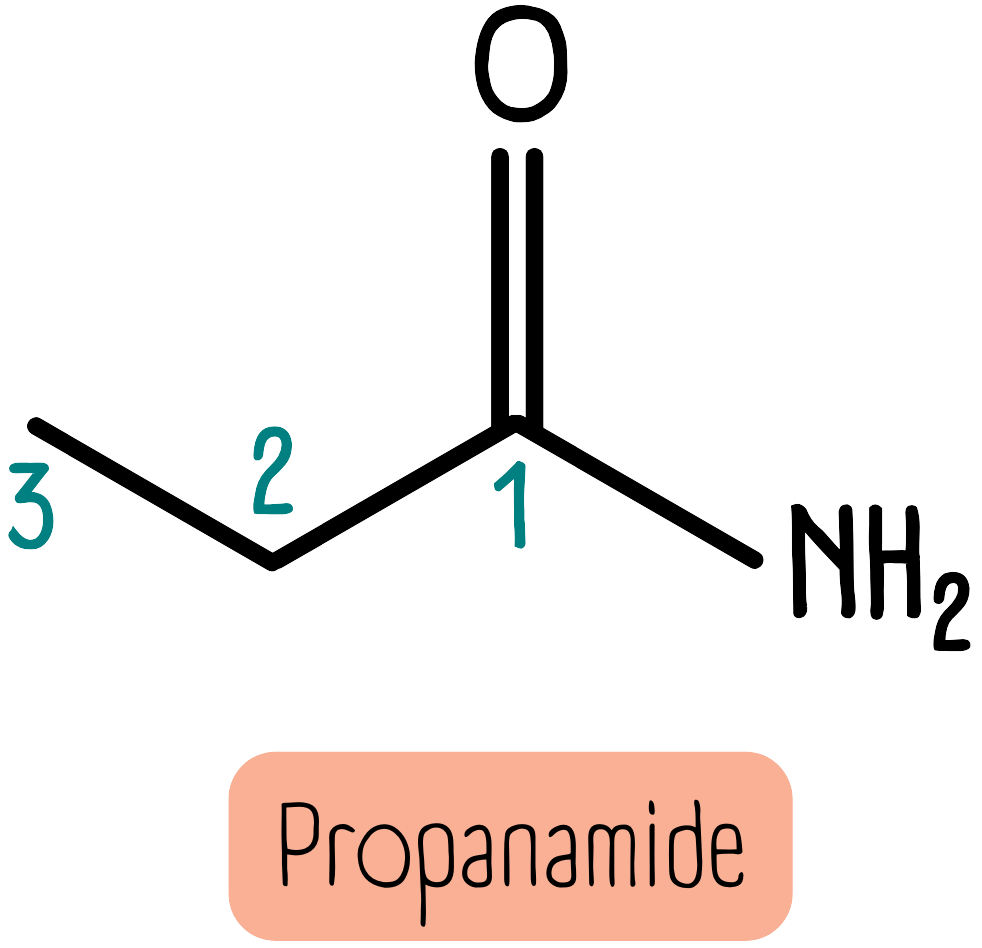
Amides are named in a similar way as amines. Primary amides have the suffix -amide. For example, the molecule below is propanamide.
Secondary and tertiary amides follow the same N- or N,N- system as above. The molecule below is N-methyl-N-propylbutanamide.
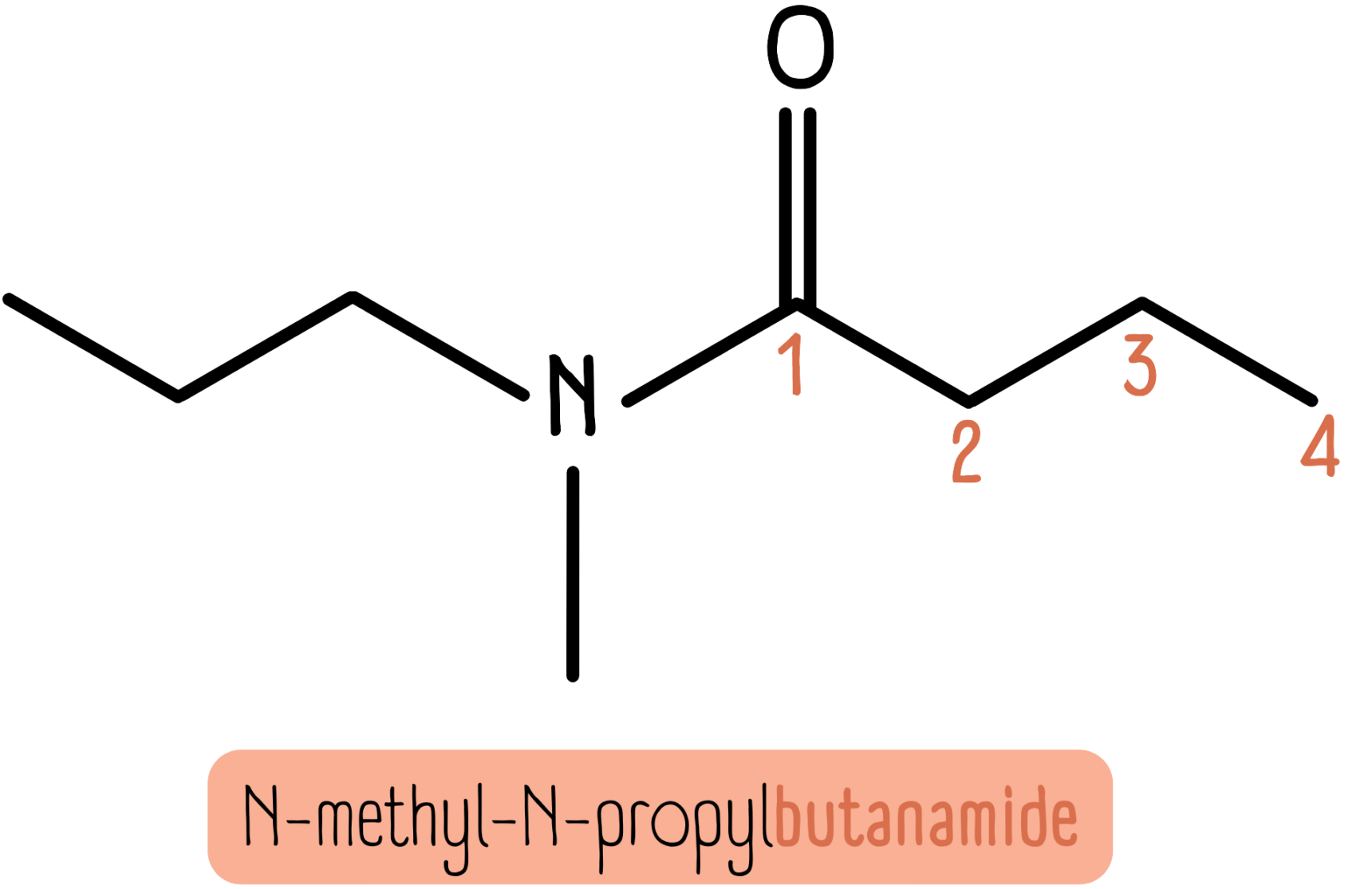
A common mistake is forgetting the carbonyl carbon when counting the chain length. In the molecule above, the amide chain consists of four carbon atoms, making it a derivative of butanamide.
Aromatics
Naming aromatic compounds is a common area of confusion. Here are the rules:
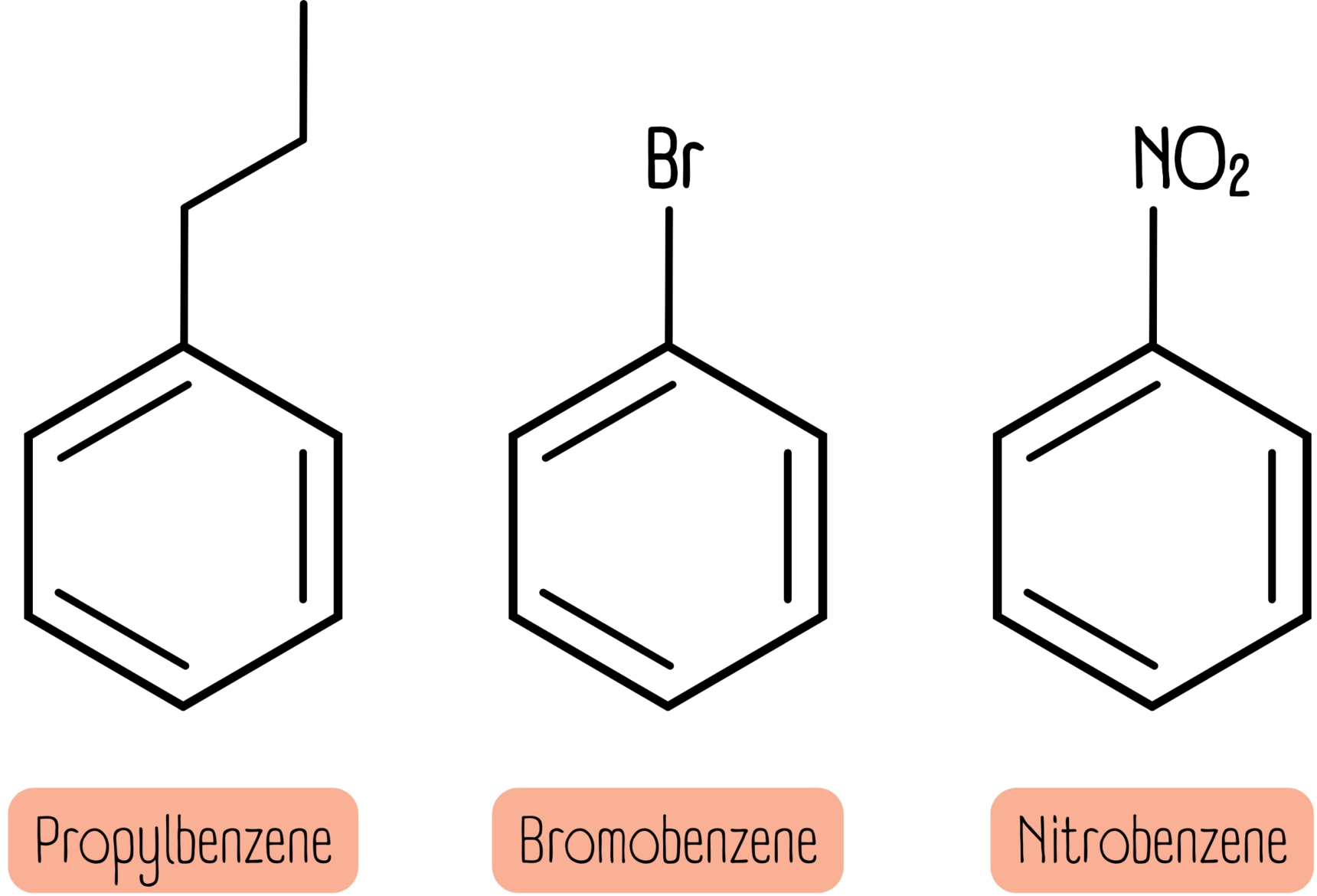
Short alkyl chains (fewer than 7 carbon atoms), halogens and nitro groups all have the suffix -benzene. Below are propylbenzene, bromobenzene and nitrobenzene.
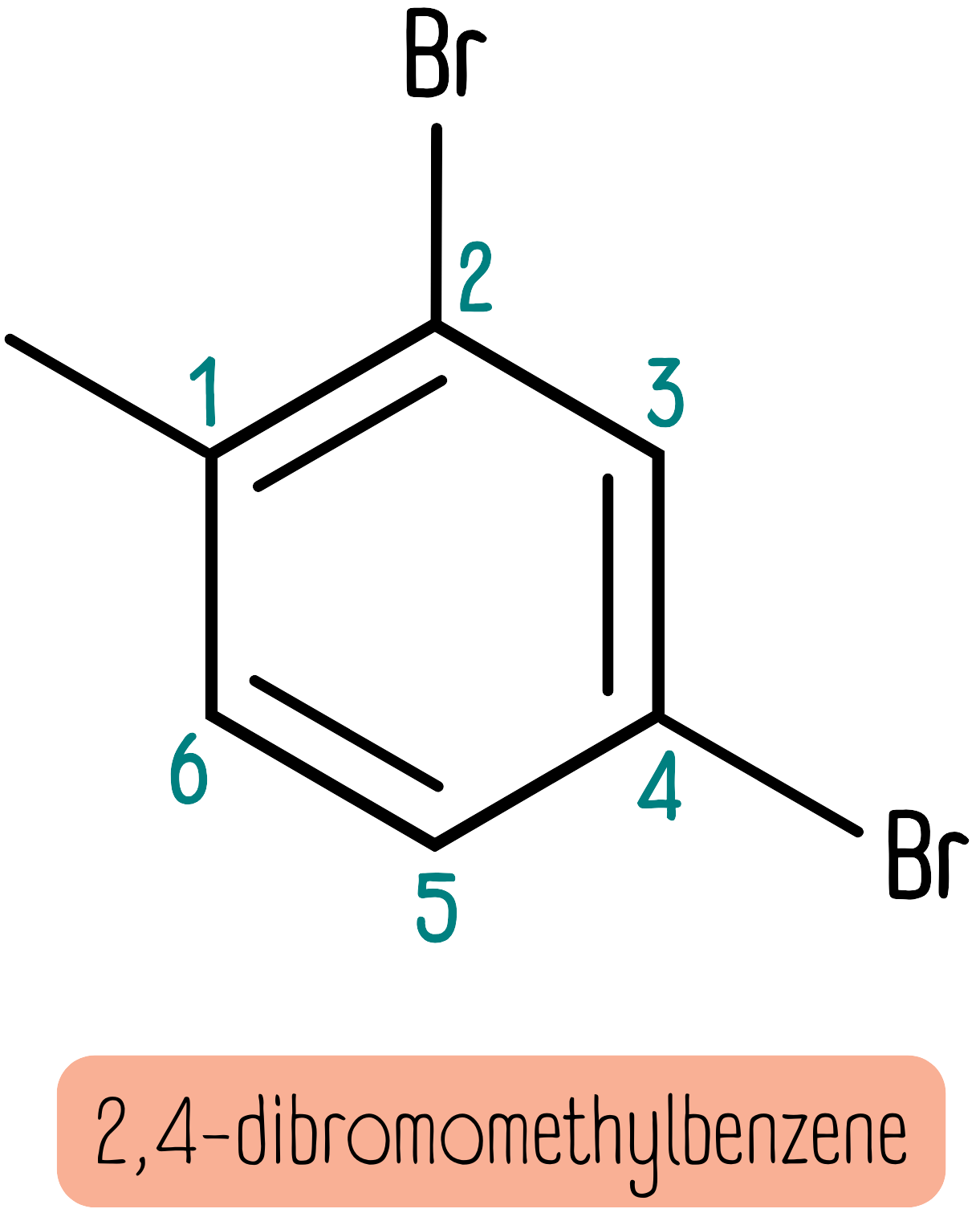
If there are multiple substituent groups, they are named using a numbering system to indicate their positions. The substituents are listed in alphabetical order, and the numbers are chosen to give the lowest possible numbers for the substituents. For example, the molecule shown below is named 2,4-dibromomethylbenzene.
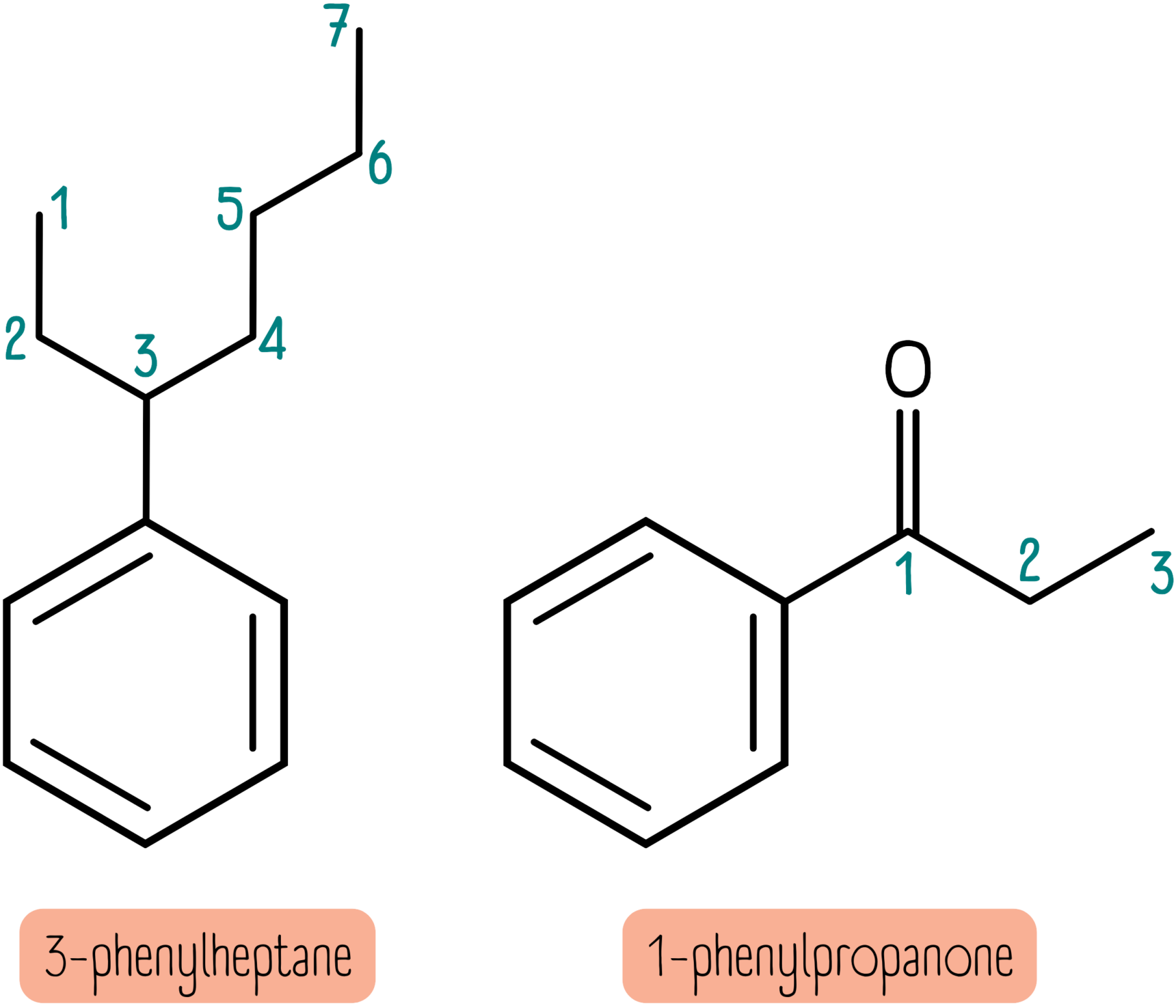
Anything more complex and the benzene ring is now treated as a phenyl group rather than the parent chain. This occurs when the alkyl chain contains another functional group or is 7 or more carbon atoms long. For instance, the molecules below are named 3-phenylheptane and 1-phenylpropanone.
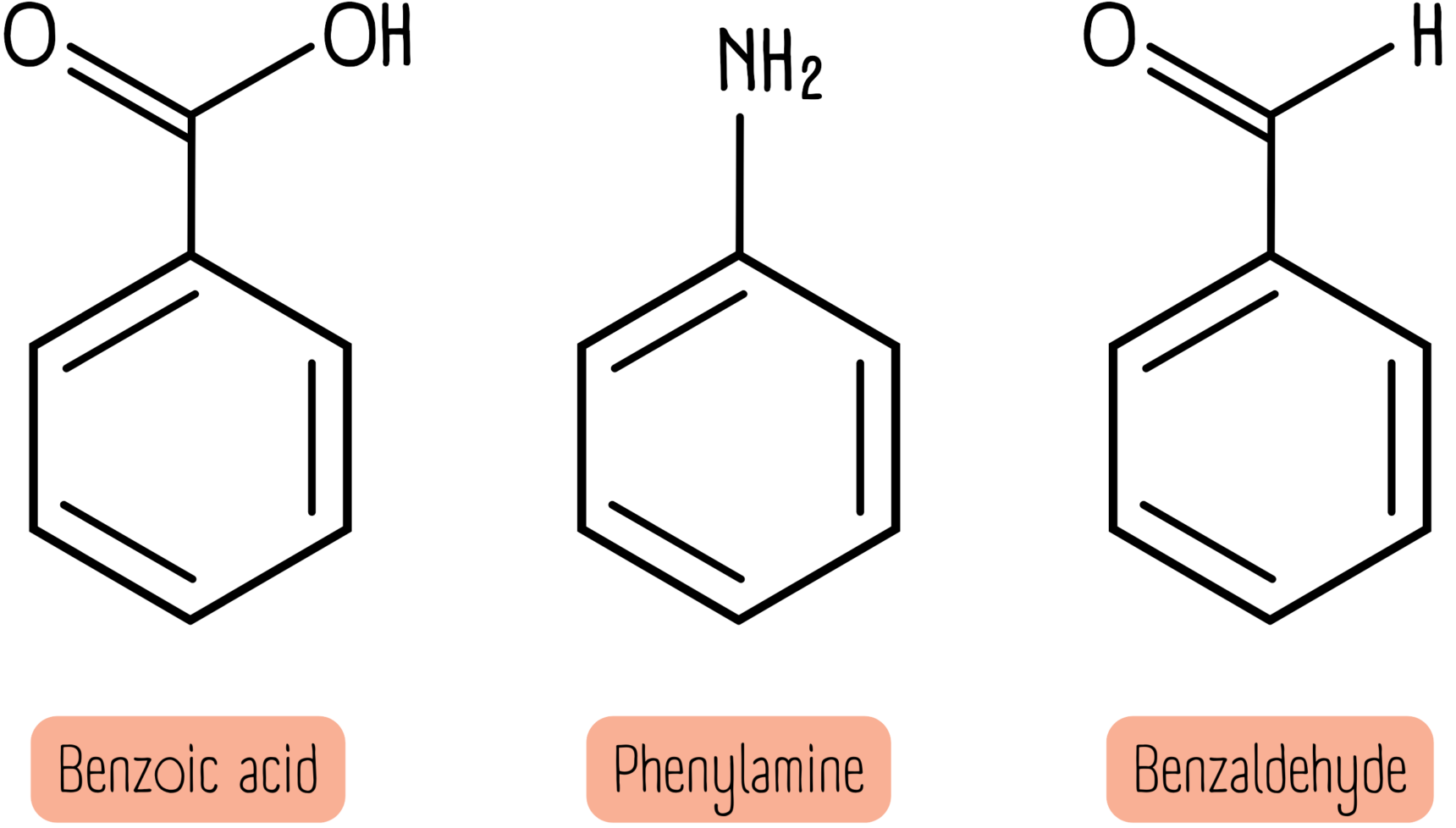
The examples below do not follow any easy to remember rules. Your best bet is to simply learn them.
Naming organic molecules may seem overwhelming, but the key is to break it down into small stages. Start with identifying the longest carbon chain to determine the base name, then look for any side chains, substituents, or functional groups, and apply the correct rules for numbering and ordering.
With practice, you’ll find that using the rules becomes second nature. Good luck!






This was really helpful for my daughter, who struggles with naming compounds. Thank you!