Contents:
Drawing organic reaction mechanisms is hard.
Why?
Because it’s like learning a new language.
But, being confident in drawing organic (or “curly arrow”) reaction mechanisms and the movement of electrons is crucial. It’s key to understanding how molecules interact and rearrange, a fundamental aspect of organic chemistry. Once you grasp the rules, you’ll be well on your way to mastering A Level Chemistry organic mechanisms.
What is a reaction mechanism?
A reaction mechanism describes the process by which reactants turn into products. One way of illustrating this is by using curly arrows to show the movement of electrons. The specific mechanisms you need to be familiar with will depend on your exam board.
What is an electrophile and what is a nucleophile?
First, we need to define two key players in reaction mechanisms: electrophiles and nucleophiles.
An electrophile is an electron-seeking substance or a species which is deficient in electrons. Electrophiles are either positively charged or polar (this could be permanent or temporary). Electrophiles will accept a pair of electrons to form a new bond.
A nucleophile is a nucleus-seeking substance or a species which is rich in electrons. Nucleophiles have a partial or full negative charge, like OH-, CN- and :NH₃. Nucleophiles will form a new bond by donating a pair of electrons.
What are the types of reaction mechanisms in organic chemistry?
This table summarises the reaction mechanisms required for most A Level Chemistry exam boards.
For comprehensive notes on each reaction mechanism tailored to your exam board, check out P&MT’s A Level Chemistry revision resources.
What does a curly arrow represent?
Curly arrows are what chemists use to show the movement of electrons. Understanding and being able to draw these arrows is essential for visualising the mechanisms of chemical reactions and the resulting molecular structures. There are some rules to follow.
What are the rules for curly arrows?
- A curly arrow represents the movement of a pair of electrons*
- The arrow shows where the electron pair starts and where it ends up
- The electron pair can come from either a bond or a lone pair
- The electron pair moves to an area lacking in electron density or is repelled from a region of electron density
- The total number of electrons should remain constant at all stages of the reaction
- The overall charge must remain constant throughout the reaction
*There is such a thing as single-headed, or fish-hook, arrows which show the movement of a single electron but these are not required in the A Level specifications for AQA, OCR, Edexcel or WJEC. These can get confusing, so it’s best to save them for when you take chemistry at uni!
Let’s look at each of these in turn.
Pairs of electrons
Most of the reactions you study will involve the movement of a pair of electrons. This is shown with a curly arrow.*
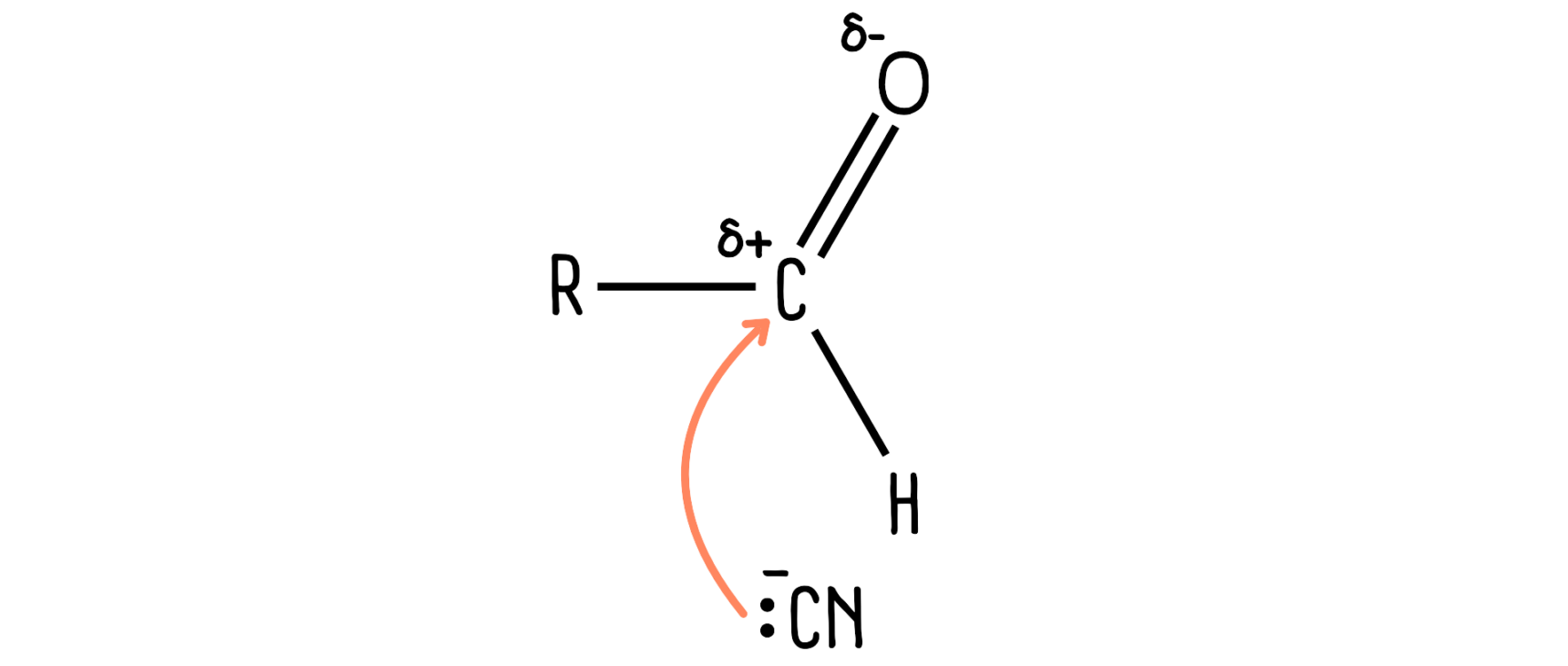
*Don’t get fixated on how curly your arrows are! You will not be penalised in the exam if your arrow is too curly, or not curly enough!
Starting point
Curly arrows must start from a source of electrons, a nucleophile. This can be either*: (1) a lone pair of electrons on an atom or (2) a bonding pair of electrons (a bond).
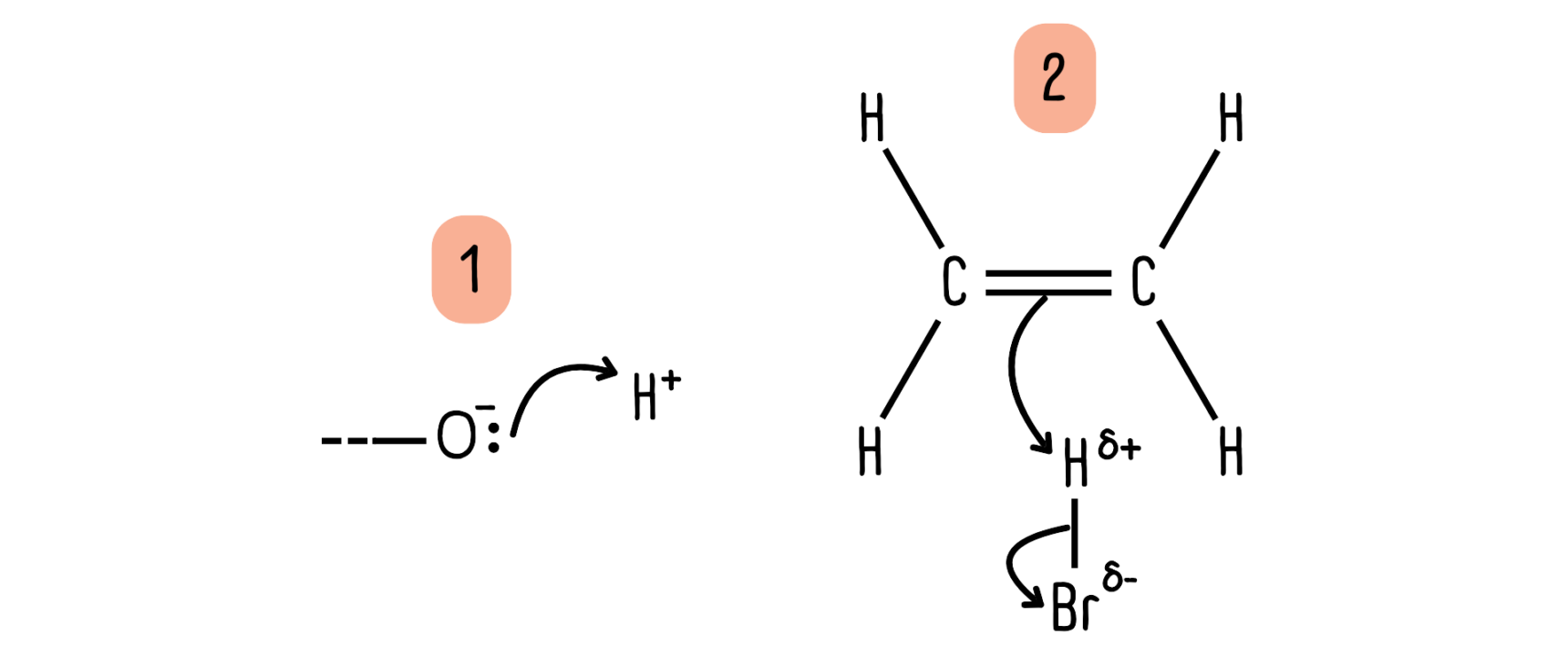
*If you are studying OCR they will also accept the arrow starting from a negative charge. If you are studying for AQA, Edexcel or WJEC, you will not get credit for this.
Ending point
Curly arrows must end at an electron-deficient site (electrophile) where a new bond is formed…
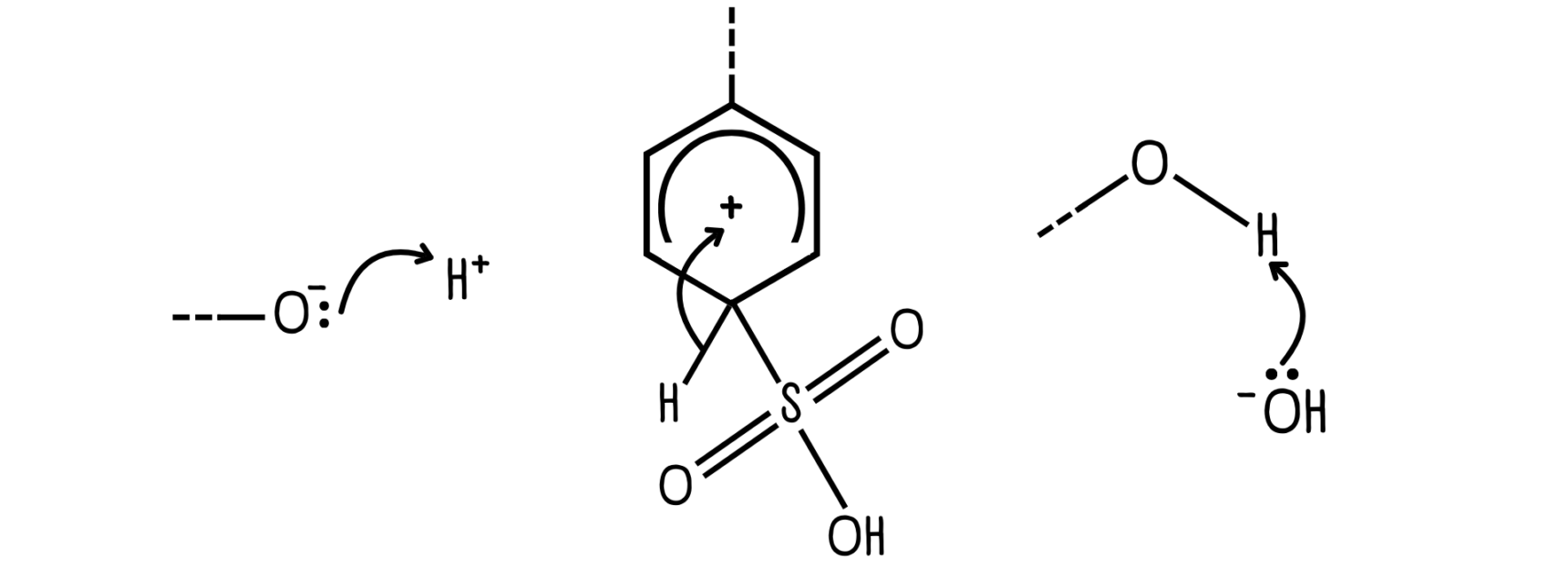
…or at a leaving group, when the leaving group takes the electrons.
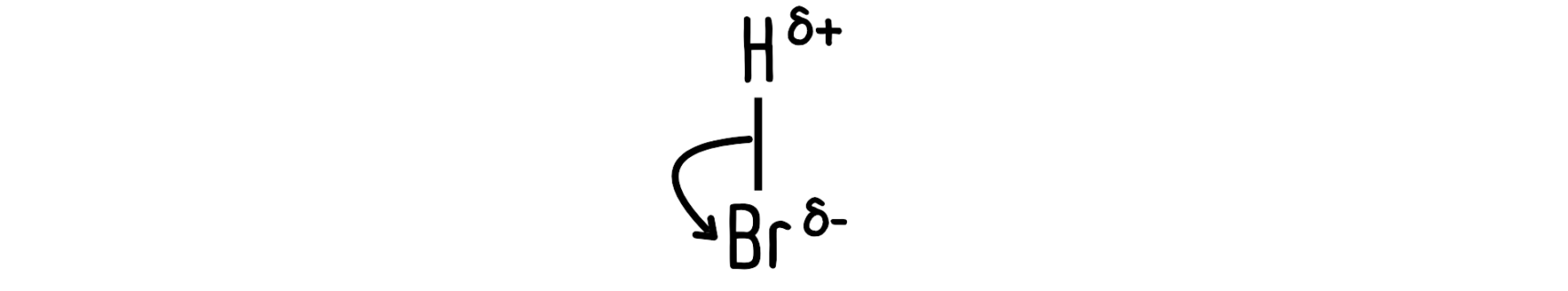
Conservation of electrons
When a bond breaks, those electrons have to go somewhere. Make sure the total number of electrons is the same on each side of the arrow.
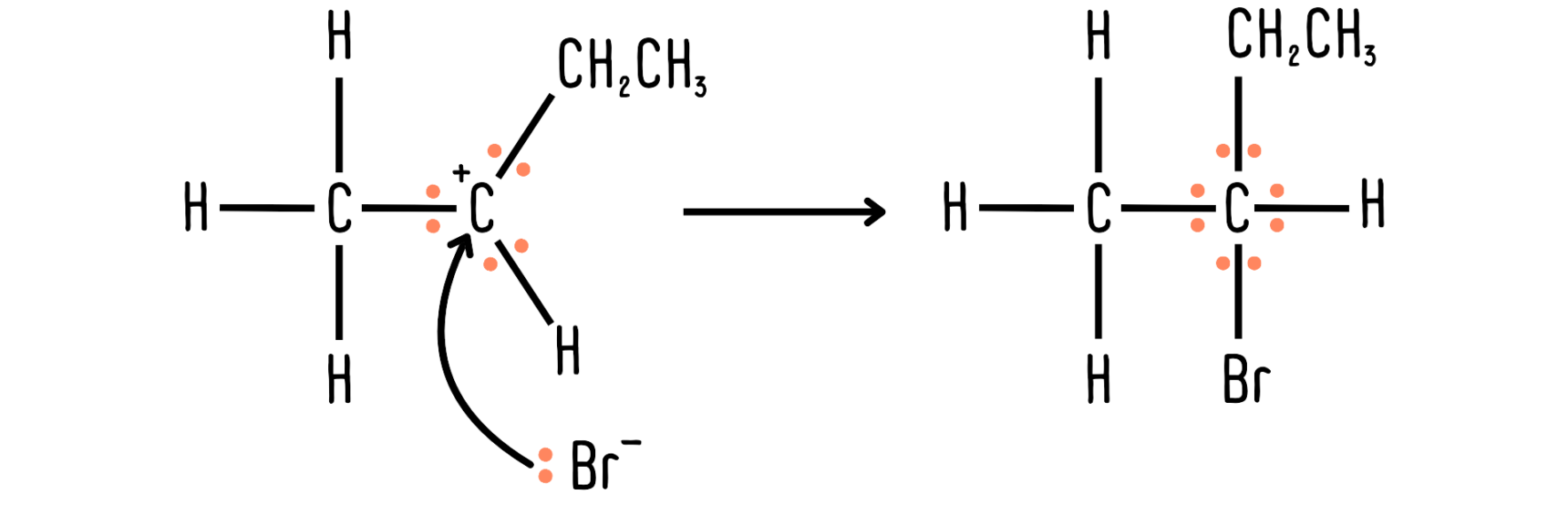
The carbon on the left has 3 bonds, so it has 6 electrons around it. 2 electrons from the lone pair on the bromide ion enter the scene, so there will be 8 electrons around the same carbon atom after the reaction.
Conservation of charge
The overall charges must be the same on either side of the reaction. In the previous example, there is a full positive and a full negative charge on the left-hand side of the reaction, so the overall charge is neutral. On the right-hand side of the reaction, there is no charge. The charges are the same on both sides of the reaction.
Reaction mechanism mistakes to avoid
There are several mistakes A Level Chemistry students commonly make when drawing reaction mechanisms:
- Drawing arrows in the wrong direction: The most common mistake students make is drawing arrows in the wrong direction. Always remember, the electrons are moving, not the atoms! The example below shows the kind of error students often make. Arrows should always begin at the source of electrons.
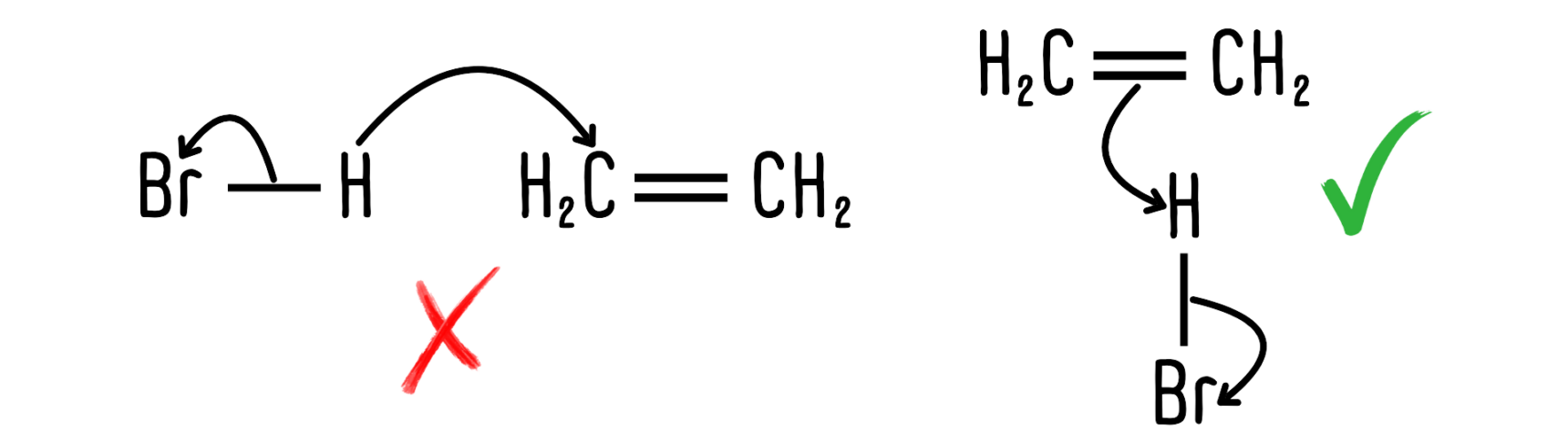
- Forgetting arrows: Missing out an arrow is easy to do, but can result in a completely incorrect product.
- Too many bonds: Always remember the number of bonds each atom ‘should’ have. For example, carbon should have 4 bonds, or a charge. Oxygen should have 2 bonds, unless it has a charge.
Practising reaction mechanisms
The best way of getting to grips with curly arrow notation is to practise practise practise! Start by finding questions that involve the same type of mechanism and you’ll quickly see common themes in the mark schemes.
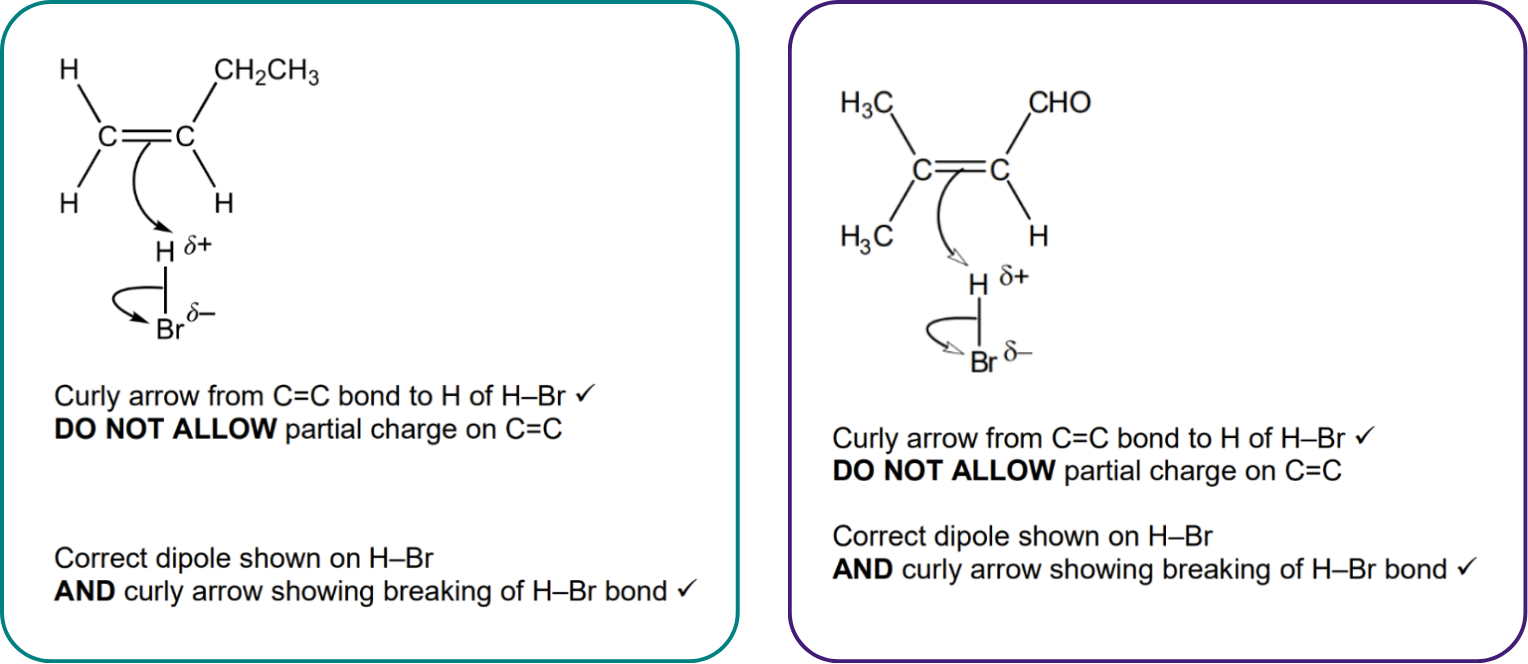
For example, here are the mark schemes for two different questions on electrophilic addition. You can see the marks are awarded for the exact same things!
How do I know which reaction mechanism to draw?
There are a few tricks here. Look carefully at the question and you should be able to spot some clues. Be careful, though, as sneaky examiners sometimes include molecules with more than one functional group!
- If the word ‘radical’ is used or UV light is mentioned, these are clues that should make you think of radical substitution.
- If there is a double bond or one of the reagents is H₂, HBr or Br₂, you should be considering electrophilic addition.
- See a halogenoalkane and aqueous hydroxide? Nucleophilic substitution. And for AQA exams, if ethanolic hydroxide is mentioned, it is definitely an elimination reaction.
- Most mechanisms involving benzene rings will be electrophilic substitution.
Concluding remarks
Drawing organic reaction mechanisms can feel like learning a new language, but getting the hang of curly arrows is key. With perseverance, you’ll build your confidence and begin to see that every arrow tells a story of how molecules change. Keep practising, and soon enough, you’ll find that this complex language starts to make sense. Good luck!



Comments