Contents:
There are so many ways of representing organic molecules that it can all get a bit confusing. Skeletal formulae are often seen as the most tricky to master, partly because they are so different from other representations. However, once understood, they become the preferred way of drawing molecules for most chemists, as they are quicker to draw, less cluttered, and make functional groups easier to identify.
Representing organic molecules
But first, what are the different ways of drawing an organic molecule?
Let’s take a look at propanone, a pretty simple molecule that can be represented in a variety of ways. In A Level Chemistry, you are expected to be able to convert between these different representations.
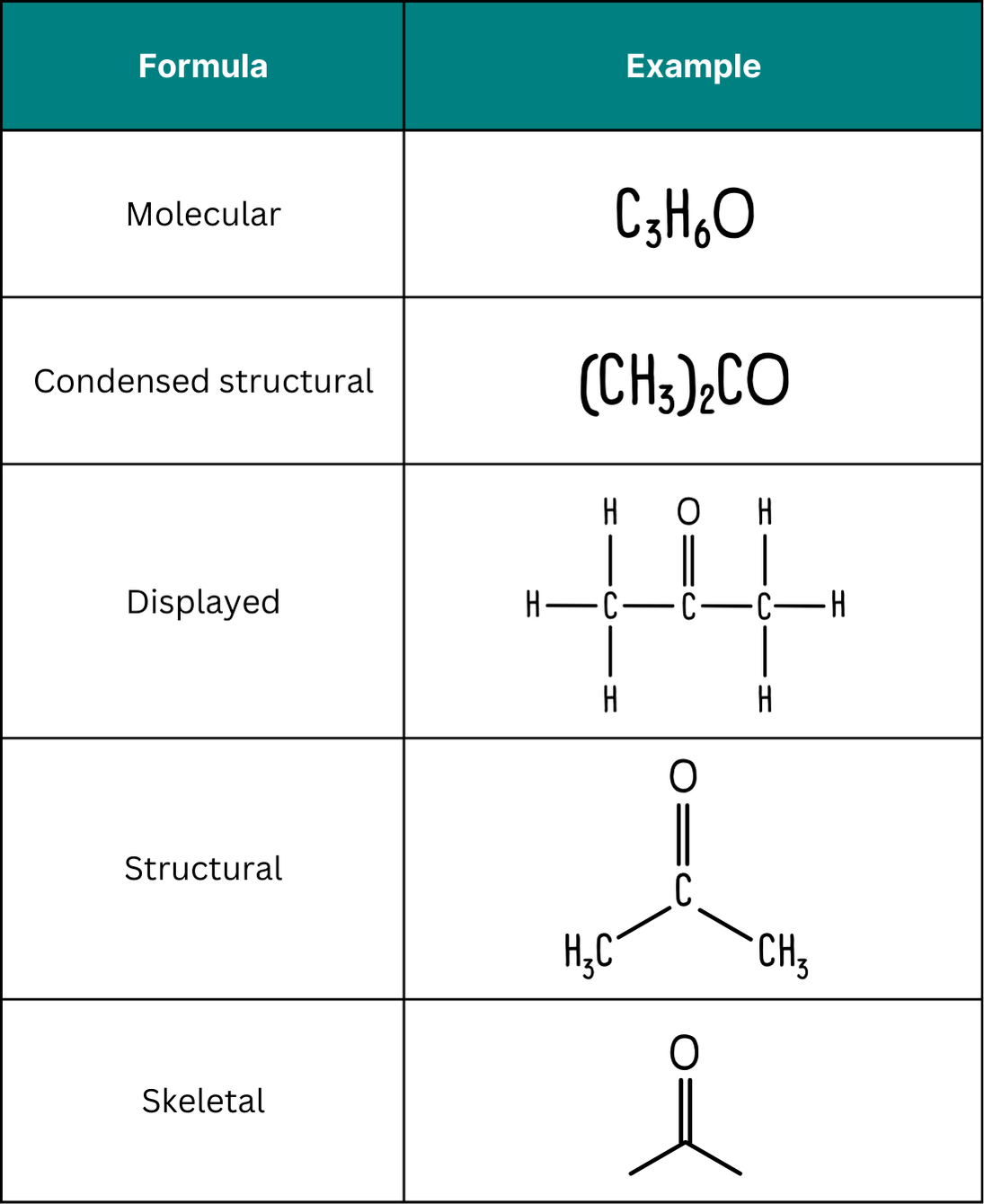
What is a skeletal formula?
A skeletal formula is a simplified displayed formula where carbon atoms are represented by the ends or vertices of lines and hydrogen atoms attached to carbon are omitted.
Students often find skeletal structures the most confusing, but once you learn how to draw them, you’ll quickly discover they’re the fastest way to sketch out a mechanism.
Basic rules for drawing skeletal structures
Like most things in chemistry, there are a few rules you need to follow!
1. Bonds and carbon atoms
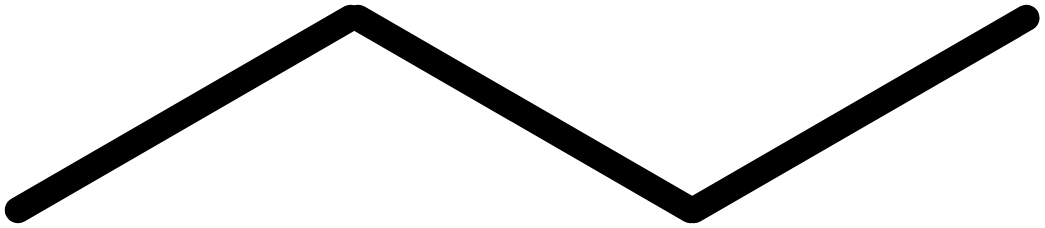
A line represents a bond, just the same as with a displayed formula. The difference lies in how we represent carbon and hydrogen atoms. In skeletal formulae, a carbon atom is assumed to be at the end of a line, or at a vertex.
So a 4 carbon chain turns from this:
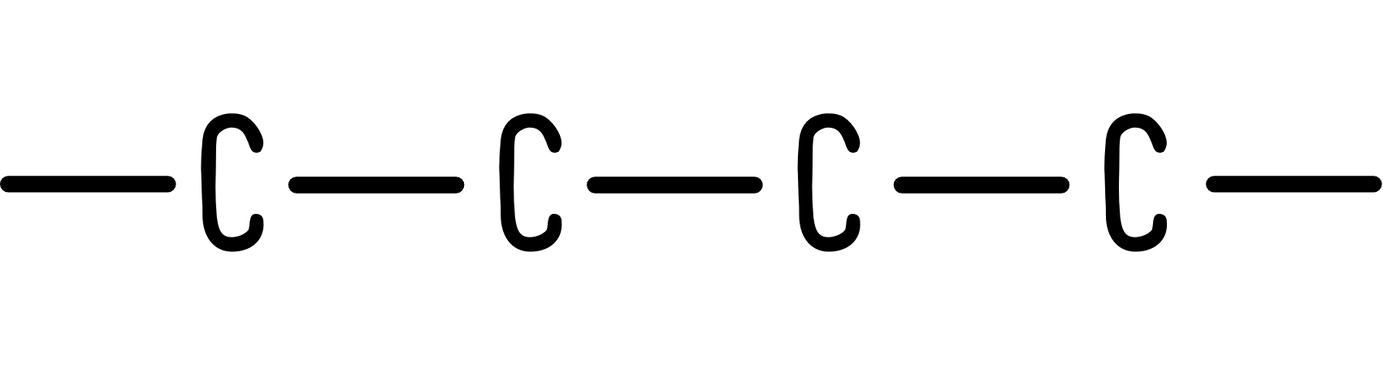
Into this:
2. Hydrogen atoms
Unless they are part of a functional group, hydrogen atoms are implied – carbon usually has 4 bonds so we assume there are enough hydrogen atoms to make up the number of bonds.
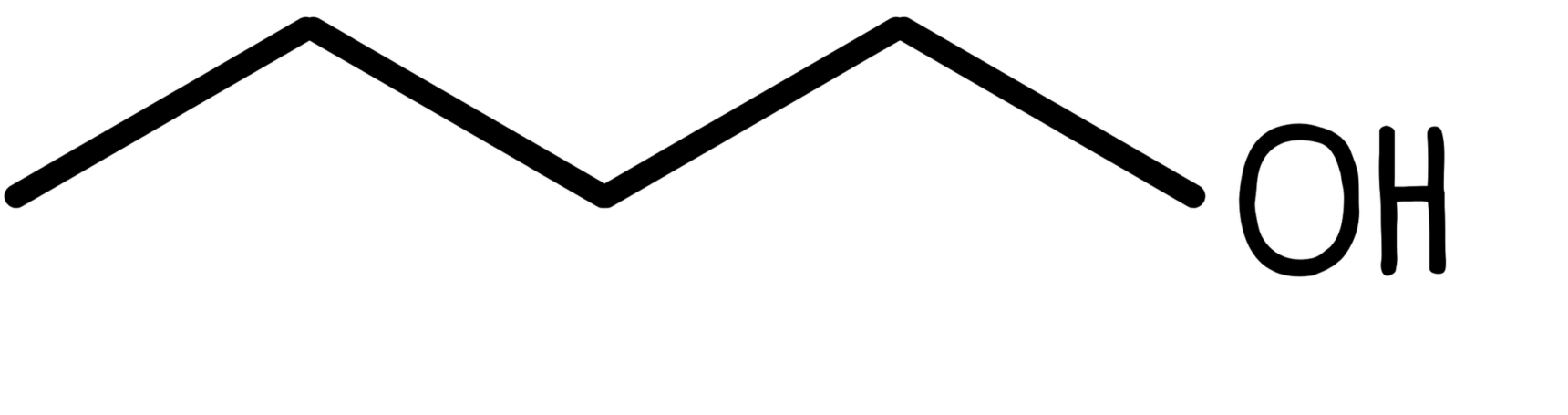
For example, butan-1-ol has 10 hydrogen atoms. Only the hydrogen atom which is part of the alcohol functional group is shown in the skeletal structure.
This:
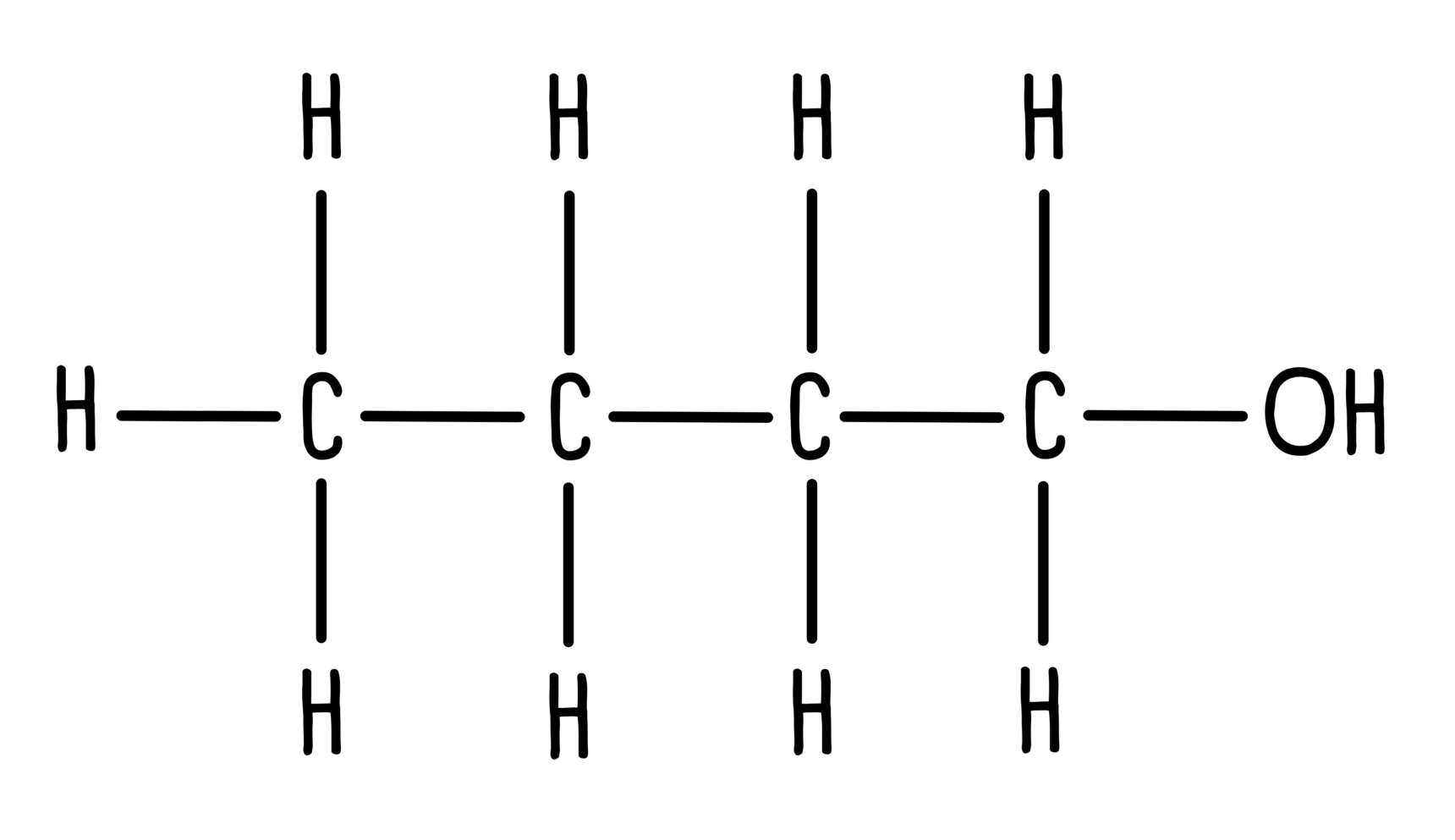
Becomes this:
3. Atoms other than carbon or hydrogen (heteroatoms)
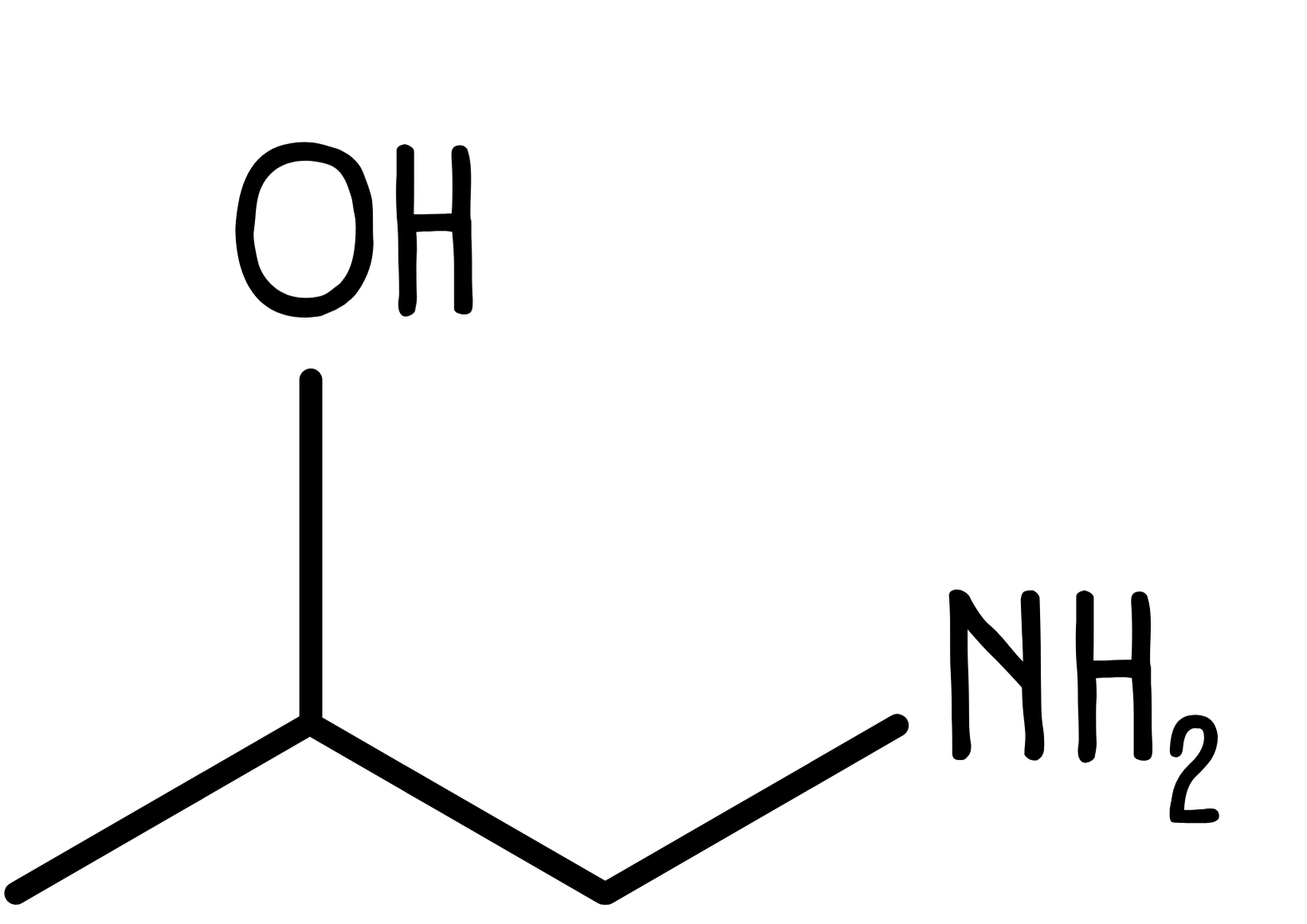
In skeletal formulae, any atom other than carbon or hydrogen is shown with its chemical symbol attached to the carbon chain.
For example, 2-hydroxypropylamine would be drawn as shown below. Note that only the hydrogen atoms in the hydroxyl (-OH) and amine (-NH₂) functional groups are drawn, while the other hydrogen atoms are implied.
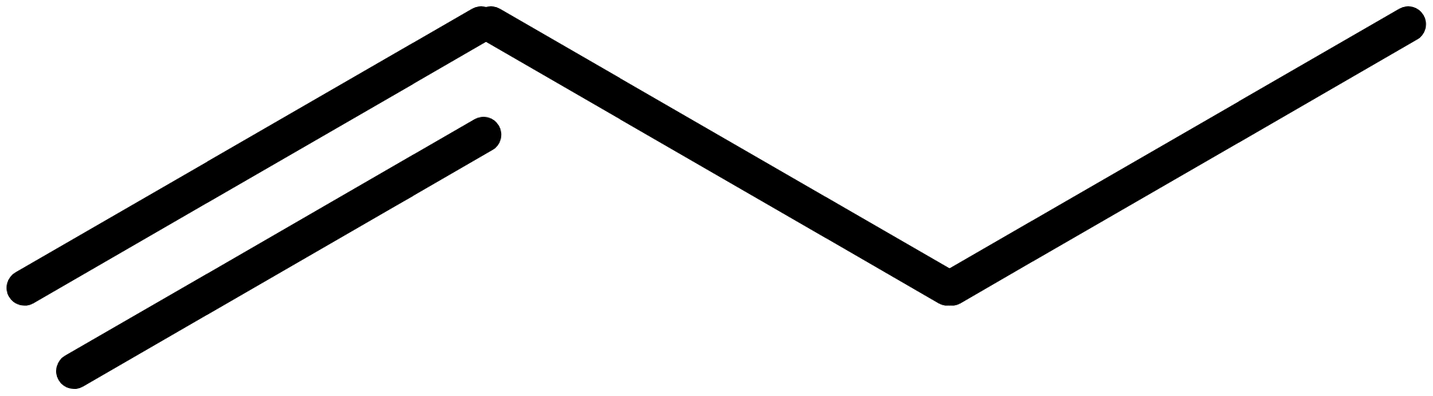
4. Multiple bonds
Double and triple bonds are shown with 2 or 3 lines, so but-1-ene is shown as:
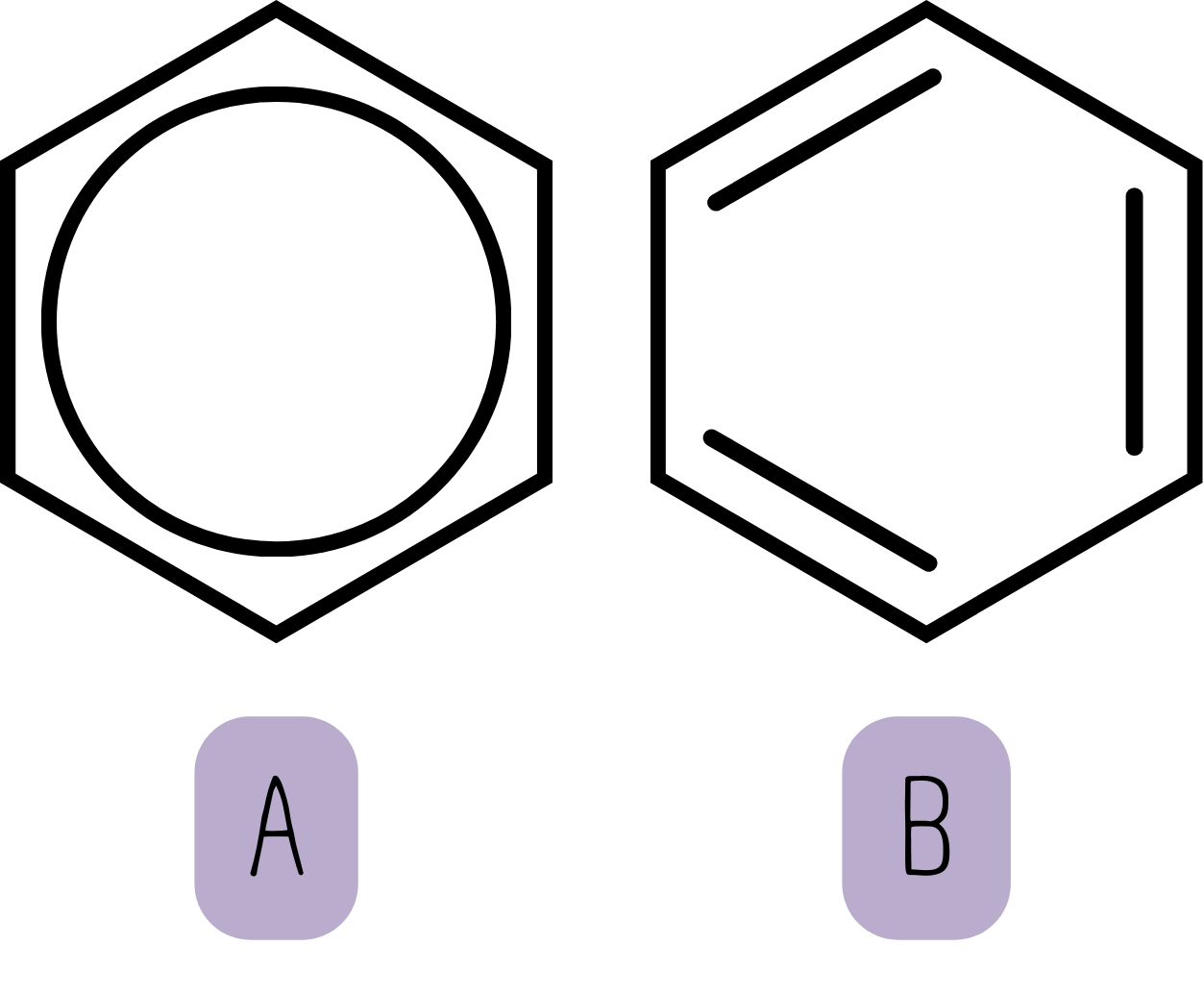
A note on benzene rings: All exam boards accept either of the following representations for benzene rings. However, it is usually better to use structure A rather than B, especially when drawing mechanisms for electrophilic substitution.
How to draw skeletal formulae
Now we have the rules, how do you draw a skeletal structure? Let’s look at some examples.
Example: 2-butenoic acid
2-butenoic acid has the displayed formula shown below. Let’s turn it into a skeletal structure.
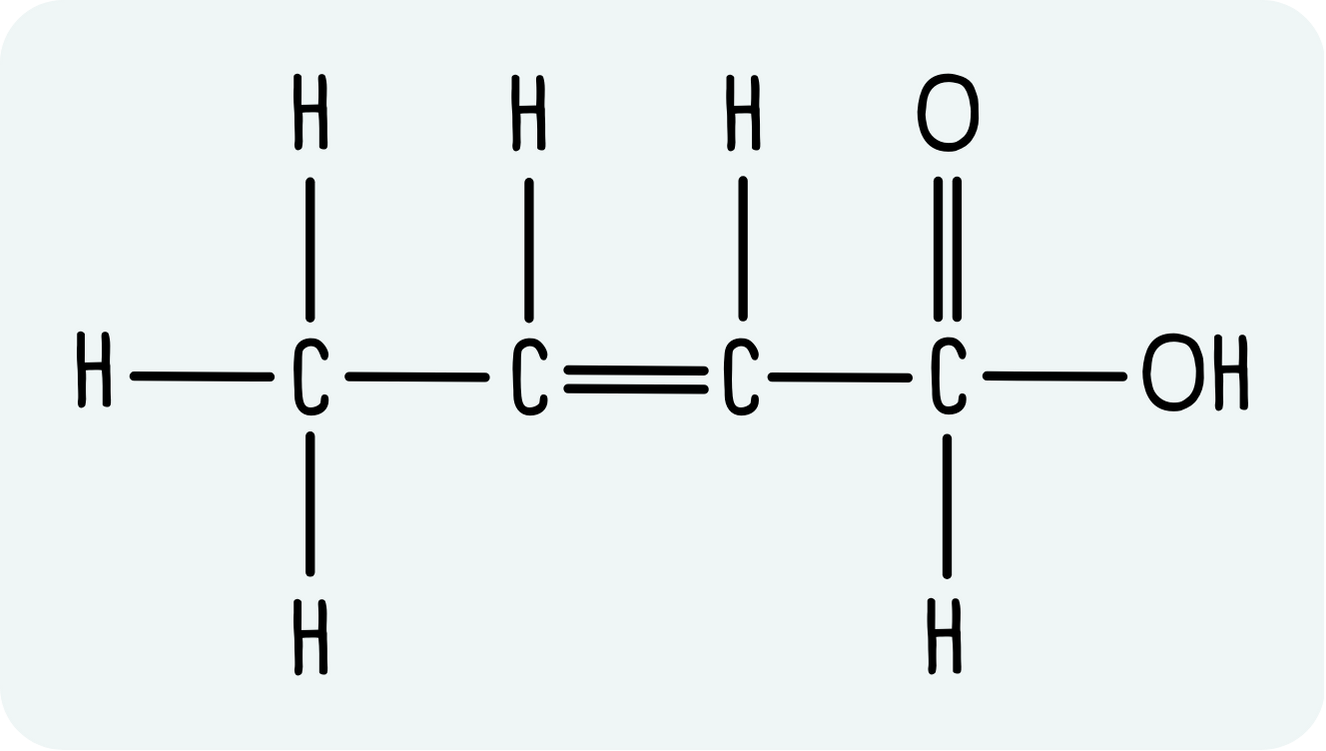
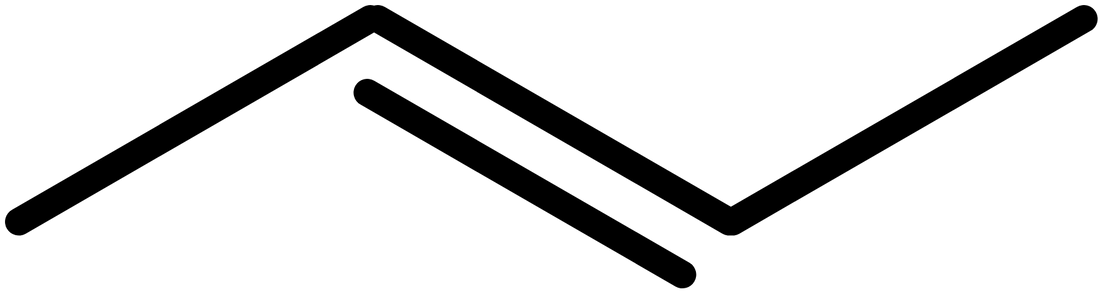
- Draw the backbone. Remember, each carbon atom is a vertex or the end of a line. So far we have:

- Add double or triple bonds. In this case, we have a double bond on the 2 position, so our structure becomes:
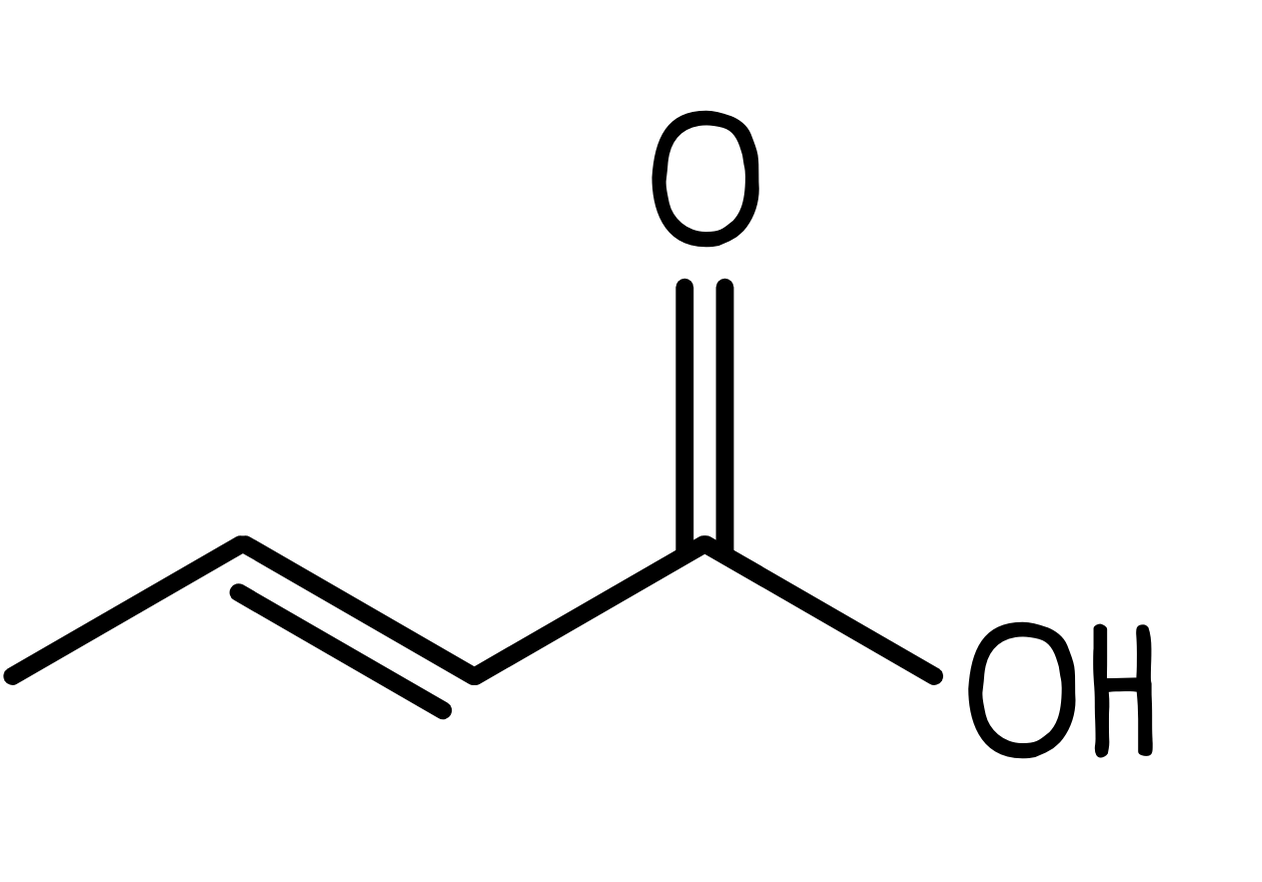
- Add functional groups. We need to add the carboxylic acid group to the end carbon, so the final structure is:
Let’s look at another example.
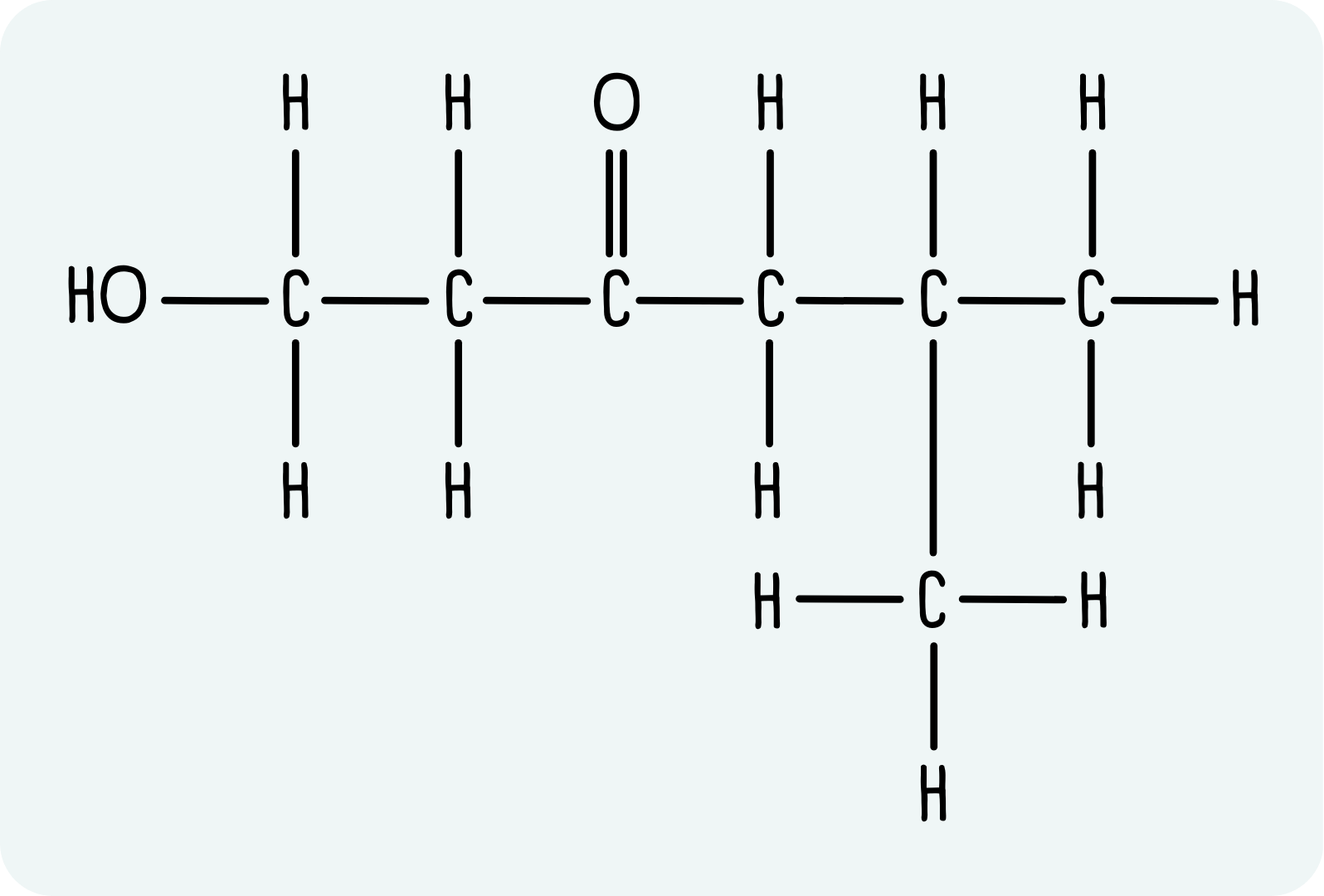
Example: 1-hydroxy-5-methylhexan-3-one
1-hydroxy-5-methylhexan-3-one has the displayed formula shown below.
- Draw the backbone. Now would also be a good time to add any side chains. This molecule has a hexyl chain with a methyl group on the 5 position:
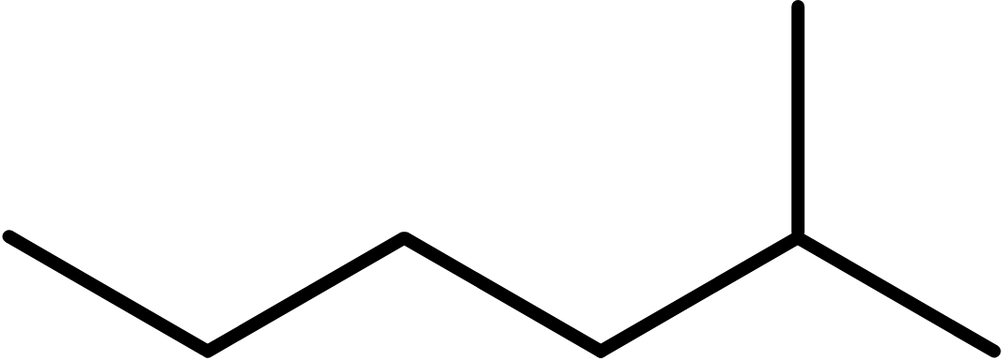
- There are no double bonds here so we move onto the next step.
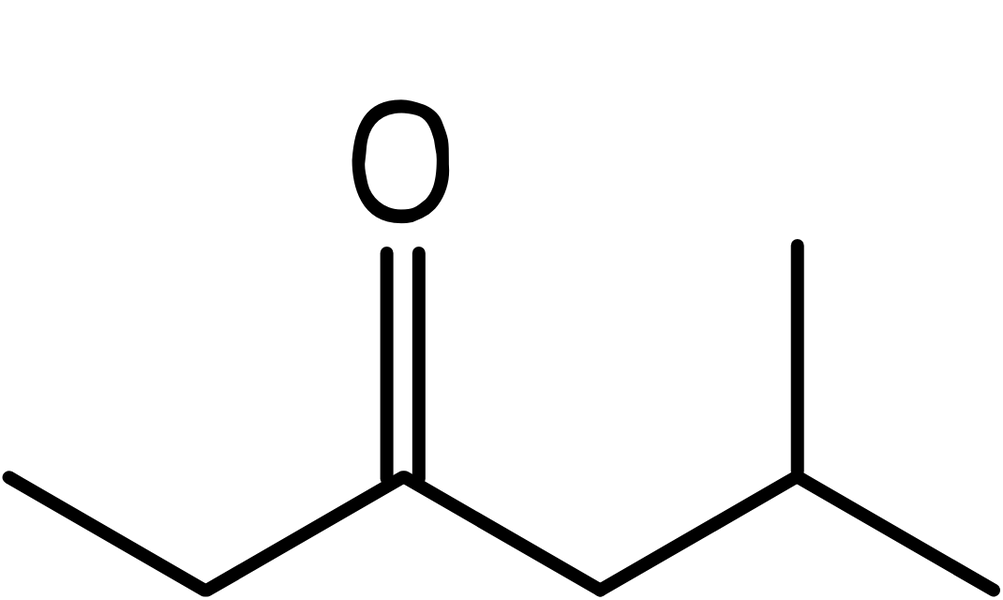
- We now add the ketone to the number 3 carbon atom giving:
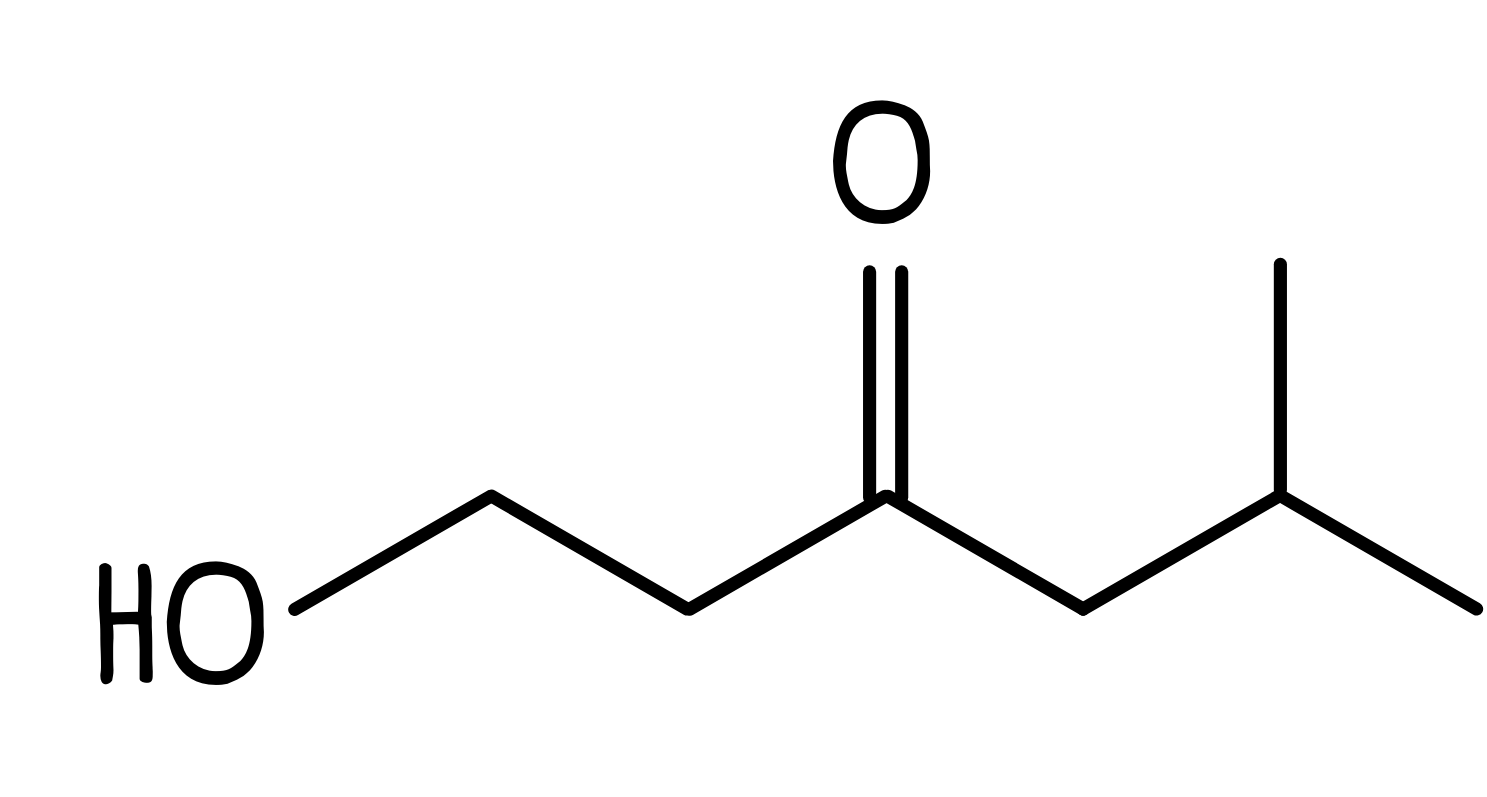
- Finally, we add the hydroxyl group to the number 1 carbon which gives us the full structure:
The best way to master skeletal formulae is to practise! You can find more revision materials and practice questions on Physics & Maths Tutor to help reinforce your understanding. With a little effort you’ll soon be confident in drawing skeletal structures. You never know, you may even prefer them!



Comments